Volume 12, Issue 3 (12-2024)
Jorjani Biomed J 2024, 12(3): 6-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pourasghari Siah Astalkhi S, Tayebi S M, Moradi L, Eslami R. The effect of eight weeks of high-intensity interval training with spirulina supplementation on apoptotic markers in the hippocampal tissue of high-fat diet-induced obese rats. Jorjani Biomed J 2024; 12 (3) :6-10
URL: http://goums.ac.ir/jorjanijournal/article-1-1042-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1042-en.html
1- Department of Sports Science, North Tehran Branch, Islamic Azad University, Tehran, Iran
2- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Tehran, Iran ,Tayebism@gmail.com
3- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Tehran, Iran
2- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Tehran, Iran ,
3- Department of Exercise Physiology, Faculty of Physical Education and Sports Sciences, Allameh Tabataba’i University, Tehran, Iran
Full-Text [PDF 550 kb]
(3188 Downloads)
| Abstract (HTML) (8310 Views)
Discussion
The hypothesis of this study was whether HIIT and Sp have a significant effect on the changes of Bax, BCL-2 gene expression and the Bax/BCL-2 in the hippocampal tissue of HFD-induced obese rats. The results showed that Bax levels and Bax/BCL-2 ratio were significantly higher in the HFD group than in the HC group. The research has shown that increasing obesity may increase the risk of Alzheimer's disease by 36%. It seems that obesity and increased fat mass are related to the decrease in the volume of gray matter in the brain, disorders in the temporal, insular, frontal cortex and other parts of the brain. In addition, the increase of ROS can lead to disruption of the immune system by reducing the power of mitochondrial biogenesis, and increasing inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in the nervous system (20). Therefore, conducting basic studies to find solutions for inhibiting inflammation, apoptosis, and cognitive impairment in obese individuals is essential.
However, the results of the present study showed that Bax levels and Bax/BCL-2 ratios in the HFD+HIIT group were significantly lower and the BCL-2 levels were significantly higher than in the HFD group. Studies show that exercises with the mechanism of increasing neurogenesis, reducing the loss of dopaminergic neurons, increasing cerebral blood flow, increasing antioxidant capacity and improving autophagy lead to improved synapse function. In other words, long-term exercises increase phosphatidyl-inositol-3 kinase (PI3K) and nuclear respiratory transcription factor 2 (NRF2), leading to increased transcription of SOD and Cat. Furthermore, long-term exercises increase neurotrophins, such as BDNF and nerve growth factor (NGF), resulting in the inhibition of procaspase-9, caspase 8, and caspase 3, and this further leads to the inhibition of tumor necrosis factor-alpha family-related ligand FASL. Inhibiting FASL prevents the release of cytochrome C, which, in turn, promotes increased expression of BCL-2 and BCL-XL as a downstream effect of these exercises. As a result, the increase in these anti-apoptotic proteins leads to a decrease in the apoptotic proteins Bax and BAK, and the Bax/BCL-2 ratio in the brain tissue (21). In addition, researchers have shown that activated PI3K after exercise leads to the activation of CREB nuclear protein, and this leads to the activation of the PI3K/AKT anti-apoptotic pathway and BDNF, pTrkB, pGRB2, and GAB1 proteins, pPI3K, and pAKT increase and this leads to the activation of the MEK/MAPK/ERK cascade (22). In this context, a study showed that aerobic training led to a decrease in the expression of death-associated protein kinase 1 (DAPK1) and FASL in the brain tissue of aged rats (23). Also, in a study, four weeks of aerobic training led to a decrease in the expression of caspase-3, MDA, and Bax, while increased BCL-2, BDNF, SOD, and Cat in the brain tissue of rats exposed to deep-heated oil (6). In another study, endurance training led to the inhibition of apoptotic proteins and the increase of anti-apoptotic proteins in the cortex tissue of diabetic rats (22). Also, in a study, exercise led to a reduction of inflammatory factors such as TNF-α, resistin, and free fatty acids in the hippocampal tissue of animals fed a high-fat diet (24). In another study, researchers compared two types of HIIT with 4-minute and 1-minute intervals and their results showed that although some anti-apoptotic adaptations in the brain tissue are dependent on the intervals, both methods of resistance training had a favorable effect on increasing the anti-apoptotic markers in the brain tissue of rats suffering from stroke (25). Therefore, from the anti-apoptotic point of view, our study confirmed the results of previous studies. Therefore, it seems that performing exercises, especially with the current research protocol, may inhibit neuronal apoptosis and consequently reduce the risk of cognitive disorders in obesity.
The results of the present study showed that in the HFD+Sp group, BCL-2 levels were significantly higher and Bax/BCL-2 levels were significantly lower than the HFD group. It seems that the main ingredient in Sp is phycocyanin-C, which can combine with peroxyl and hydroxyl free radicals in the brain tissue as soon as possible, prevent the activity of lipid peroxidation, and ultimately lead to the modulation of the immune system in different parts of the brain (26). For example, in a study, researchers showed that Sp, in addition to having non-enzymatic antioxidants such as vitamin B6 and B12, with the mechanism of reducing free radicals, can lead to the inhibition of inflammatory factors and the reduction of TNF-α, 6-hydroxydopamine (6-OHDA) and MDA, as well as increases SOD, GPx, nitric oxide (NO), thereby improving antioxidant capacity (27). In another study, it was stated that Sp, by reducing lipopolysaccharides, leads to a decrease in inflammation, an increase in the expression of BDNF, activation of phosphorylated protein kinase A (pAKT), a decrease in cooxygenase-2 (COX-2), an increase in NRF2, p-cAMP, and p-CREB leads to the transcription of metabolic genes and increasing the expression of BCL-2 in brain tissue (28). In this context, researchers found that 500 mg/kg of Sp for 14 days led to an increase in SOD, GPx, Ca, and a decrease in IL-1 and IL-6 in the brain tissue of rats exposed to methotrexate (29).
The results showed that in the HFD+HIIT+Sp group, Bax and Bax/BCL-2 levels were significantly lower, whereas BCL-2 levels were significantly higher than the HFD group. In the context of the simultaneous effect of exercise and Sp on brain tissue, a study showed that six weeks of resistance training and Sp simultaneously improved antioxidants, reduced oxidative stress, improved cognitive function, and increased SOD in the hippocampal tissue of rats exposed to stanozolol (14). It seems that exercise with the mechanism of activation of PI3K/NRF2 signaling, increase of expression of SOD and Cat, increase of BDNF and NGF, activation of PI3K/AKT/CEREB signaling, leads to inhibition of procaspase-9, caspase 8, caspase 3, FASL, decrease of BAX, BAK, release of cytochrome C, increase the expression of BCL-2 and BCL-XL (21,22). Also, Sp with the mechanism of neutralizing free radicals, reducing lipid peroxidation (26), inhibition of TNF-α, 6-OHDA, and MDA, increase of SOD, GPx, and NO (Saraswathi, 2023), increase of BDNF, activation of pAKT, decrease of COX-2, increase of NRF2, p-cAMP, and p-CREB, leads to the transcription of metabolic genes, increasing the expression of BCL-2 and decreasing Bax (27). Therefore, the information shows that exercise and Sp supplement are effective in improving neuronal function and reducing apoptosis in brain tissue. Since Bax and Bax/BCL-2 levels were significantly lower and BCL-2 levels significantly higher in the HFD+HIIT and HFD+HIIT+Sp groups compared to the HFD+Sp group. Exercises that more effectively target lipid metabolism through catecholamine pathways and enhance cAMP phosphorylation appear to have a stronger impact than Sp (16). In this regard, the results of a study showed that the combination of aerobic exercise and Sp on increasing nesfatin 1 and neuropeptide gamma was more favorable than the effect of Sp (16). Also, according to the results of the present study, Bax levels were significantly lower, and BCL-2 levels were significantly higher in the HFD+HIIT+Sp group than HFD+HIIT group. It seems that exercise and Sp supplement probably strengthen each other's effect and have a synergistic effect in reducing neuronal apoptosis through common pathways. Consistent with our findings, Aghahi et al. demonstrated that the combined effect of exercise and Sp more effectively reduced miR125b and miR146a (Markers of oxidative stress) and improved cognitive function compared to either intervention alone (14). In addition to examining the effects of the combination of exercise and Sp in human samples, a study also showed that HIIT with Sp led to an increase in VO2 peak, omentin-1, irisin, spexin and a decrease in lipocalin-2 and asprosin and improved the body composition of obese men (30). In another study by the same researchers, the combination of HIIT and Sp training led to the improvement of fat profile, reduction of TNF-α, C-reactive protein (CRP), Sema3C, IL-6, monocyte protein-1 (MCP-1) and IL-8 in obese men (30). Also, in a study, researchers showed that aerobic exercise and Sp simultaneously increased Nesfatin-1 and Peptide YY in overweight elderly men (16). Therefore, it seems that the combination of these two interventions, both clinically and in experimental studies on animals, can improve metabolic and nervous system disorders.
Considering the role of oxidative stress and antioxidants in the promotion of apoptosis, the lack of measurement of these variables was one of the limitations of the present study. Therefore, it is suggested to evaluate oxidant-antioxidant markers in future studies. Also, considering the role of neurotrophins in inhibiting apoptosis, which was not measured in the present study, it is suggested to evaluate neurotrophins in future studies. In addition, it seems that the inability to generalize the results to human samples, different methods of measuring variables and considering different exercise intensities are other limitations of the present study. Therefore, it is suggested that these limitations be used as research manipulations in future studies.
Conclusion
It seems that HIIT and Sp supplement separately have a beneficial effect on some apoptotic markers in hippocampal tissue in obese rats. However, their interactive effect on inhibiting neuronal apoptosis in brain tissue in obesity conditions is more obvious than the effect of each one alone.
Acknowledgement
This manuscript is a part of a doctoral degree by Soraya Pourasghari Siah Astalkhi, a doctoral student at Azad University of North Tehran. The research assistant of this academic unit is hereby appreciated. We also acknowledge the animal laboratory expert of Islamic Azad University, Marvdasht branch, Omidreza Salehi, for his guidance and help.
Funding sources
The authors received no financial support from any organization.
Ethical statement
All the ethical principles of working with animals in this research were carried out under the supervision of the Ethics Committee in Biomedical Research of the Islamic Azad University, Sanandaj branch, with the approval no. IR.IAU.SDJ.REC.1401.099.
Conflicts of interest
The authors declared no conflicts of interest.
Author contributions
In this manuscript, Soraya Pourasghari Siah Astalkhi has been responsible for financial support, data preparation, and practical research protocol. The initial writing of the manuscript was done by Seyed Morteza Tayibi, Soraya Pourasghari Siah Astalkhi, and Rasul Islami. The research implementation was supervised by Seyed Morteza Tayibi and Lida Moradi. Seyed Morteza Tayibi was also the final editor of the manuscript.
Full-Text: (1645 Views)
Introduction
Obesity is still one of the most common metabolic disorders in human societies. According to researchers, weight gain and obesity are related to the occurrence of many diseases, such as increased cholesterol, blood pressure, type 2 diabetes mellitus, gall bladder disease, arthritis, sleep disorders, and various cancers (1). The occurrence of obesity occurs as a result of an imbalance in calorie intake and calorie consumption (1,2). Obesity causes disturbance in lipid profile, disturbance in glucose, and insulin metabolism (3). As a result, an imbalance of free fatty acids leads to an increase in reactive oxygen species (ROS) by increasing ATP production and the activity of the electron transport chain (4). Therefore, with the occurrence of obesity, leptin and adiponectin, which regulate appetite control, are disturbed in the brain tissue and eventually the occurrence of cognitive disorders (4). More specifically, the increase in oxidative stress and inflammatory markers in the brain tissue following obesity (5) leads to the activation of apoptotic markers, such as caspases, protein X associated with B cell lymphoma-2 (Bax), and the decrease of neurotrophins and the protein of B cell lymphoma-2 (BCL-2) in the brain tissue (6).
On the other hand, due to the increasing awareness of the complications of obesity, the use of exercises for weight loss has been introduced as the best method along with a proper diet (6). Researchers have shown that regular and long-term exercises can lead to weight loss, improve mobility and reduce the risks of obesity by improving lipid metabolism, glucose and insulin metabolism (2,3,7). In this context, a meta-analysis study showed that endurance training for four and eight weeks led to an increase in superoxide dismutase (SOD), and catalase (Cat); however, as the intensity of the training increased at the end, the activity of antioxidants also increased and this indicated the high level of ROS in the brain tissue (8). In an original study, the researchers showed that aerobic training for four weeks and 40 minutes per session led to a decrease in BAX, Casp-3, Casp-9, malondialdehyde (MDA) and glial fibrillary acidic protein (GFAP) in the hippocampal tissue of rats exposed to deep-frying oil (6). In another review study, the researchers stated that although regular, moderate-intensity aerobic exercise enhances antioxidant activity in the brain, intense, exhausting anaerobic, and combined forms of exercise may raise oxidative stress levels in this tissue (9). Therefore, the information about the role of exercise on the antioxidant system and apoptosis in the brain tissue is still not well known.
On the other hand, due to the introduction of exercise as the most desirable way to lose weight, researchers have recently used natural supplements and antioxidants to reduce weight (10) and neuronal disorders following metabolic diseases (11). Among these supplements, spirulina (Sp) is known as an antioxidant supplement that is known as a cyanobacterium rich in protein, polyunsaturated fatty acids, C-phycocyanin and other biologically active compounds. Sp has been recommended for obesity and overweight due to its antioxidant, anti-inflammatory and improving glucose and lipid metabolism properties (12). Improving cerebral blood flow and increasing the activity of SOD, GPx, and Cat, Sp supplement can lead to an increase in brain-derived neurotrophic factor (BDNF) as well as receptor tyrosine kinase B (TrkB) in the brain tissue, and in this way lead to an improvement in cognitive function (13). Also, Sp supplementation led to an increase in SOD, GPx, improvement in cognitive function, and an increase in the number of healthy cells in the C1 and C3 areas of the hippocampus following oxidative damage induced by abuse of anabolic steroids (14). It is noteworthy that Sp supplementation leads to a decrease in Bax and an increase in BCL-2 by inhibiting inflammatory factors and oxidative stress (15).
In addition, the use of this antioxidant, along with exercises also, improves antioxidants, cognitive function (14), gamma neuropeptide, and nesfatin (16) in conditions of obesity and metabolic disorders. Although there have been studies on the effect of exercise on improving brain function, the effect of exercise with different intensities, especially High-Intensity Interval Training (HIIT), on the mechanism of apoptosis in the brain tissue following obesity is still unknown. Considering the effects of exercise on apoptotic mechanisms, it seems that the use of an antioxidant such as Sp may provide a better understanding of the mechanism of neuronal apoptosis following obesity and a suitable solution for obese people to lose weight. Therefore, we hypothesized that the combination of HIIT and Sp would synergistically reduce apoptosis in the hippocampal tissue of high-fat diet-(HFD)-induced obese rats."
Methods
Animals housing
This research was an experimental study with a post-test design along with a control group. In this research, 30 male Sprague-Dawley rats, weighing 200.41 ± 20.11, were purchased from the animal house of the Islamic Azad University, Marvdasht branch, and kept in the sports physiology laboratory of this university for one week for familiarization with the new environment. During the research, the standard conditions of keeping animals including 12-hour cycle of light and darkness, temperature of 22±3 °C and humidity of 55-60% were observed. Also, during the research period, rats were kept in polycarbonate washable cages. Sterile wood shavings were used to absorb the moisture in the cages. In addition, during the period, the rats were kept in a quiet environment. Then, 24 rats were fed a diet of 60% fat, 20% protein, and 20% carbohydrate (Each gram of this diet contains 5.24 kcal) for eight weeks to reach a weight of over 310 grams (17). Next, the obese rats were randomly divided into four groups of six rats, including 1) HFD, 2) HFD+Sp, 3) HIIT+HFD, and 4) HFD+HIIT+Sp. In addition, six healthy rats were included in the healthy control group (HC) to investigate the effects of HFD and obesity on research variables. During eight weeks, groups 3 and 4 performed HIIT for three sessions per week on a treadmill for animals, and groups 2 and 4 received Sp supplements at a dose of 100 mg/kg orally dissolved in drinking water.
Training protocol
For HIIT drills in this research, the rats were familiarized with treadmill by running on a treadmill for one week (Five minutes daily at a speed of 8 m/m). Then the rats warmed up for five minutes at a speed of 8 m/min in one session to measure the maximum running speed, and then their speed was increased by 3 m/m for every three minutes until they reached exhaustion. It is worth noting that in this research, exhaustion was defined as a state in which rats hit the end of the treadmill three times in a row in less than one minute (2). To implement the main research protocol, high-intensity intervals were set at two minutes each. During these intervals, intensity was adjusted weekly: 80% of maximum speed in the first week, 90% in the second, 100% in the third, and 110% from the fourth week onward until the end of training. Each high-intensity interval was followed by a two-minute low-intensity recovery period at 50% of maximum speed. After performing the last repetition of the high-intensity interval, the rats performed the cooling down for five minutes with an intensity of 50-60% of the maximum speed. The number of high-intensity interval repetitions was determined according to the training week of the male rats. The first week consisted of two high-intensity interval repetitions; the second week consisted of four high-intensity interval repetitions; the third week consisted of six high-intensity interval repetitions; and from the beginning of the fourth week onwards, there were eight high-intensity interval repetitions. Therefore, the total training time including repetition of high-intensity and low-intensity intervals along with warm-up and cool-down was 16 minutes on average in the first week, 24 minutes in the second week, 32 minutes in the third week, and 40 minutes from the beginning of the fourth week onwards (18). It is worth noting that this training model is primarily based on animal training models to evaluate the anti-apoptotic effects of HIIT. In addition, this training method can be used in the future as a suitable method for human training to achieve more success in a short time.
Spirulina supplement
Spirulina supplement was purchased from Merck Company (Germany with economic code 724424-92-4). Then 10 grams of Sp powder was mixed in a sterile container with 100 ml of water at 17°C with a magnetic stirrer for 10 minutes and then the solution was kept at 4°C for 24 hours. Next, Sp supplement in the amount of 100 mg/kg in milliliter bottles was fed to rats (19). It is worth mentioning that based on previous studies, the dose used in this research had favorable effects on the heart and nervous system in the condition of obesity.
Sampling
At the end of the research, 48 hours after the last training session and in a 12-hour fasting state in order to remove the acute effect of training and Sp supplementation and any effect of diet, rats were injected intra-peritoneal with ketamine (70 mg/kg) and xylazine (20 mg/kg) purchased from Alfasan Company (Netherlands). To ensure unconsciousness, foot, stomach and tail pinch tests were used, and after ensuring complete unconsciousness, the hair on the upper part of the skull was shaved first; then, using a surgical cutter and scissors, the skull cavity was split and the brain tissue was carefully extracted. The brain tissue was immediately placed in the tissue storage chamber and transferred to -70 °C temperature.
Bax and BCL-2 measurement method
For the gene expression levels of Bax and BCL-2, RNA was first extracted from the hippocampal tissue according to the manufacturer's protocol (FavorPrep™ Tissue Total RNA Mini Kit: Hong Kong). For this purpose, the homogenized tissue was transformed into a homogenous solution using RB Buffer along with β-mercaptoethanol. The solution was kept in the room for five minutes. Then it was centrifuged for two minutes. Next, the centrifuged solution was vortexed with 350 µl of 70% alcohol. Then the solution was centrifuged again for one minute. Next, the solution was vortexed with 350 µl of 70% alcohol. Then the solution was centrifuged again for one minute. This step was repeated twice, then the RB Mini Column was placed in the Elution Tube and 50 µl of RNase-free ddH2O was added to the RB Mini Column for one minute, and then centrifuged for two minutes at a speed of 14,000 rpm. In this step, RNA was extracted. Then by using the property of light absorption at a wavelength of 260 nm and with the help of the following formula, the concentration and degree of purity of RNA was obtained quantitatively.
C (µg/µl) = A260×ɛ×d/1000
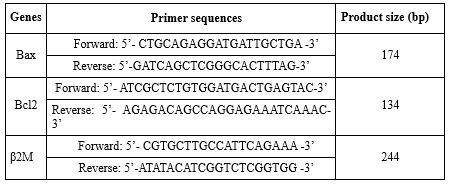
After extracting RNA with very high purity and concentration from all samples, cDNA synthesis steps were performed according to the manufacturer's protocol Fermentas (Cat No: K162), and then the synthesized cDNA was used to perform the reverse transcription reaction. For this purpose, 1,000 ng of RNA, 0.5 microliter of Random Hexamers, 0.5 oligodT primer, 1 microliter of RiboLock RNase Inhibitor, 1 microliter of RevertAid™ M-MuLV Reverse transcriptase, 4 microliters of 5X Reaction Buffer, 2 microliters of DNTP and 20 microliters of DEPC-treated water was used. All steps were performed according to the Fermentas kit brochure mentioned for cDNA synthesis. Next, the solution and designed primers were designed with Allele IDv7.8 software. To consider the internal control gene, beta 2 myoglobulin gene (β2m) was used. Next, the housekeeping gene solution, primers and cDNA were placed in the wells of the qReal-time PCR machine made in Italy named StepOne and the machine started working. Then, the solution continued to work using the device at different times and temperatures until the gene expression reached the threshold (Cycle Threshold). Also, 2-ΔΔCT method was used to quantify the gene expression levels of the data. Also, the internal control gene was considered Beta 2 Microglobulin (B2M) in this research. The sequence of primers used in the study is presented in Table 1.
Obesity is still one of the most common metabolic disorders in human societies. According to researchers, weight gain and obesity are related to the occurrence of many diseases, such as increased cholesterol, blood pressure, type 2 diabetes mellitus, gall bladder disease, arthritis, sleep disorders, and various cancers (1). The occurrence of obesity occurs as a result of an imbalance in calorie intake and calorie consumption (1,2). Obesity causes disturbance in lipid profile, disturbance in glucose, and insulin metabolism (3). As a result, an imbalance of free fatty acids leads to an increase in reactive oxygen species (ROS) by increasing ATP production and the activity of the electron transport chain (4). Therefore, with the occurrence of obesity, leptin and adiponectin, which regulate appetite control, are disturbed in the brain tissue and eventually the occurrence of cognitive disorders (4). More specifically, the increase in oxidative stress and inflammatory markers in the brain tissue following obesity (5) leads to the activation of apoptotic markers, such as caspases, protein X associated with B cell lymphoma-2 (Bax), and the decrease of neurotrophins and the protein of B cell lymphoma-2 (BCL-2) in the brain tissue (6).
On the other hand, due to the increasing awareness of the complications of obesity, the use of exercises for weight loss has been introduced as the best method along with a proper diet (6). Researchers have shown that regular and long-term exercises can lead to weight loss, improve mobility and reduce the risks of obesity by improving lipid metabolism, glucose and insulin metabolism (2,3,7). In this context, a meta-analysis study showed that endurance training for four and eight weeks led to an increase in superoxide dismutase (SOD), and catalase (Cat); however, as the intensity of the training increased at the end, the activity of antioxidants also increased and this indicated the high level of ROS in the brain tissue (8). In an original study, the researchers showed that aerobic training for four weeks and 40 minutes per session led to a decrease in BAX, Casp-3, Casp-9, malondialdehyde (MDA) and glial fibrillary acidic protein (GFAP) in the hippocampal tissue of rats exposed to deep-frying oil (6). In another review study, the researchers stated that although regular, moderate-intensity aerobic exercise enhances antioxidant activity in the brain, intense, exhausting anaerobic, and combined forms of exercise may raise oxidative stress levels in this tissue (9). Therefore, the information about the role of exercise on the antioxidant system and apoptosis in the brain tissue is still not well known.
On the other hand, due to the introduction of exercise as the most desirable way to lose weight, researchers have recently used natural supplements and antioxidants to reduce weight (10) and neuronal disorders following metabolic diseases (11). Among these supplements, spirulina (Sp) is known as an antioxidant supplement that is known as a cyanobacterium rich in protein, polyunsaturated fatty acids, C-phycocyanin and other biologically active compounds. Sp has been recommended for obesity and overweight due to its antioxidant, anti-inflammatory and improving glucose and lipid metabolism properties (12). Improving cerebral blood flow and increasing the activity of SOD, GPx, and Cat, Sp supplement can lead to an increase in brain-derived neurotrophic factor (BDNF) as well as receptor tyrosine kinase B (TrkB) in the brain tissue, and in this way lead to an improvement in cognitive function (13). Also, Sp supplementation led to an increase in SOD, GPx, improvement in cognitive function, and an increase in the number of healthy cells in the C1 and C3 areas of the hippocampus following oxidative damage induced by abuse of anabolic steroids (14). It is noteworthy that Sp supplementation leads to a decrease in Bax and an increase in BCL-2 by inhibiting inflammatory factors and oxidative stress (15).
In addition, the use of this antioxidant, along with exercises also, improves antioxidants, cognitive function (14), gamma neuropeptide, and nesfatin (16) in conditions of obesity and metabolic disorders. Although there have been studies on the effect of exercise on improving brain function, the effect of exercise with different intensities, especially High-Intensity Interval Training (HIIT), on the mechanism of apoptosis in the brain tissue following obesity is still unknown. Considering the effects of exercise on apoptotic mechanisms, it seems that the use of an antioxidant such as Sp may provide a better understanding of the mechanism of neuronal apoptosis following obesity and a suitable solution for obese people to lose weight. Therefore, we hypothesized that the combination of HIIT and Sp would synergistically reduce apoptosis in the hippocampal tissue of high-fat diet-(HFD)-induced obese rats."
Methods
Animals housing
This research was an experimental study with a post-test design along with a control group. In this research, 30 male Sprague-Dawley rats, weighing 200.41 ± 20.11, were purchased from the animal house of the Islamic Azad University, Marvdasht branch, and kept in the sports physiology laboratory of this university for one week for familiarization with the new environment. During the research, the standard conditions of keeping animals including 12-hour cycle of light and darkness, temperature of 22±3 °C and humidity of 55-60% were observed. Also, during the research period, rats were kept in polycarbonate washable cages. Sterile wood shavings were used to absorb the moisture in the cages. In addition, during the period, the rats were kept in a quiet environment. Then, 24 rats were fed a diet of 60% fat, 20% protein, and 20% carbohydrate (Each gram of this diet contains 5.24 kcal) for eight weeks to reach a weight of over 310 grams (17). Next, the obese rats were randomly divided into four groups of six rats, including 1) HFD, 2) HFD+Sp, 3) HIIT+HFD, and 4) HFD+HIIT+Sp. In addition, six healthy rats were included in the healthy control group (HC) to investigate the effects of HFD and obesity on research variables. During eight weeks, groups 3 and 4 performed HIIT for three sessions per week on a treadmill for animals, and groups 2 and 4 received Sp supplements at a dose of 100 mg/kg orally dissolved in drinking water.
Training protocol
For HIIT drills in this research, the rats were familiarized with treadmill by running on a treadmill for one week (Five minutes daily at a speed of 8 m/m). Then the rats warmed up for five minutes at a speed of 8 m/min in one session to measure the maximum running speed, and then their speed was increased by 3 m/m for every three minutes until they reached exhaustion. It is worth noting that in this research, exhaustion was defined as a state in which rats hit the end of the treadmill three times in a row in less than one minute (2). To implement the main research protocol, high-intensity intervals were set at two minutes each. During these intervals, intensity was adjusted weekly: 80% of maximum speed in the first week, 90% in the second, 100% in the third, and 110% from the fourth week onward until the end of training. Each high-intensity interval was followed by a two-minute low-intensity recovery period at 50% of maximum speed. After performing the last repetition of the high-intensity interval, the rats performed the cooling down for five minutes with an intensity of 50-60% of the maximum speed. The number of high-intensity interval repetitions was determined according to the training week of the male rats. The first week consisted of two high-intensity interval repetitions; the second week consisted of four high-intensity interval repetitions; the third week consisted of six high-intensity interval repetitions; and from the beginning of the fourth week onwards, there were eight high-intensity interval repetitions. Therefore, the total training time including repetition of high-intensity and low-intensity intervals along with warm-up and cool-down was 16 minutes on average in the first week, 24 minutes in the second week, 32 minutes in the third week, and 40 minutes from the beginning of the fourth week onwards (18). It is worth noting that this training model is primarily based on animal training models to evaluate the anti-apoptotic effects of HIIT. In addition, this training method can be used in the future as a suitable method for human training to achieve more success in a short time.
Spirulina supplement
Spirulina supplement was purchased from Merck Company (Germany with economic code 724424-92-4). Then 10 grams of Sp powder was mixed in a sterile container with 100 ml of water at 17°C with a magnetic stirrer for 10 minutes and then the solution was kept at 4°C for 24 hours. Next, Sp supplement in the amount of 100 mg/kg in milliliter bottles was fed to rats (19). It is worth mentioning that based on previous studies, the dose used in this research had favorable effects on the heart and nervous system in the condition of obesity.
Sampling
At the end of the research, 48 hours after the last training session and in a 12-hour fasting state in order to remove the acute effect of training and Sp supplementation and any effect of diet, rats were injected intra-peritoneal with ketamine (70 mg/kg) and xylazine (20 mg/kg) purchased from Alfasan Company (Netherlands). To ensure unconsciousness, foot, stomach and tail pinch tests were used, and after ensuring complete unconsciousness, the hair on the upper part of the skull was shaved first; then, using a surgical cutter and scissors, the skull cavity was split and the brain tissue was carefully extracted. The brain tissue was immediately placed in the tissue storage chamber and transferred to -70 °C temperature.
Bax and BCL-2 measurement method
For the gene expression levels of Bax and BCL-2, RNA was first extracted from the hippocampal tissue according to the manufacturer's protocol (FavorPrep™ Tissue Total RNA Mini Kit: Hong Kong). For this purpose, the homogenized tissue was transformed into a homogenous solution using RB Buffer along with β-mercaptoethanol. The solution was kept in the room for five minutes. Then it was centrifuged for two minutes. Next, the centrifuged solution was vortexed with 350 µl of 70% alcohol. Then the solution was centrifuged again for one minute. Next, the solution was vortexed with 350 µl of 70% alcohol. Then the solution was centrifuged again for one minute. This step was repeated twice, then the RB Mini Column was placed in the Elution Tube and 50 µl of RNase-free ddH2O was added to the RB Mini Column for one minute, and then centrifuged for two minutes at a speed of 14,000 rpm. In this step, RNA was extracted. Then by using the property of light absorption at a wavelength of 260 nm and with the help of the following formula, the concentration and degree of purity of RNA was obtained quantitatively.
C (µg/µl) = A260×ɛ×d/1000
After extracting RNA with very high purity and concentration from all samples, cDNA synthesis steps were performed according to the manufacturer's protocol Fermentas (Cat No: K162), and then the synthesized cDNA was used to perform the reverse transcription reaction. For this purpose, 1,000 ng of RNA, 0.5 microliter of Random Hexamers, 0.5 oligodT primer, 1 microliter of RiboLock RNase Inhibitor, 1 microliter of RevertAid™ M-MuLV Reverse transcriptase, 4 microliters of 5X Reaction Buffer, 2 microliters of DNTP and 20 microliters of DEPC-treated water was used. All steps were performed according to the Fermentas kit brochure mentioned for cDNA synthesis. Next, the solution and designed primers were designed with Allele IDv7.8 software. To consider the internal control gene, beta 2 myoglobulin gene (β2m) was used. Next, the housekeeping gene solution, primers and cDNA were placed in the wells of the qReal-time PCR machine made in Italy named StepOne and the machine started working. Then, the solution continued to work using the device at different times and temperatures until the gene expression reached the threshold (Cycle Threshold). Also, 2-ΔΔCT method was used to quantify the gene expression levels of the data. Also, the internal control gene was considered Beta 2 Microglobulin (B2M) in this research. The sequence of primers used in the study is presented in Table 1.
Data analysis method
Mean and standard deviation were used for descriptive statistics. Shapiro-Wilk test was used to check the normality of data distribution. Next, according to the normality of the data distribution, one-way analysis of variance (ANOVA) along with Tukey's post-hoc tests and two-way ANOVA along with Bonferonie's post-hoc tests were used for analyzing data in the SPSS software version 20. A significance level of 0.05 was considered for all analyses.
Results
The results of one-way ANOVA test showed a significant difference in the gene expression levels of Bax (F = 23.31 and P-Value = 0.001), BCL-2 (F = 115.83 and P-Value = 0.001) and Bax/BCL-2 (F = 313.22 and P-Value = 0.001) in research groups. The results of Tukey's post-hoc test showed that the Bax levels in the HFD group were significantly higher than the HC group (P-Value = 0.016). No significant difference was observed in the HFD and HFD+Sp groups (P-Value = 0.34); however, in the HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups, Bax levels were significantly lower than the HFD group, also in the HFD+HIIT (P-Value = 0.006) and HFD+HIIT+Sp (P-Value = 0.001) groups were significantly lower than the HFD+Sp group as well as in the HFD+HIIT+Sp group were significantly lower than the HFD+HIIT group (P-Value = 0.04). The results of the two-way ANOVA showed that the HIIT (P-Value = 0.001, F = 68.05 and Eta: 0.77) and Sp (P-Value = 0.004, F = 10.27 and Eta: 0.33) had a significant effect on the reduction of Bax gene expression in the hippocampal tissue of HFD-induced obese rats. Nevertheless, the interaction of HIIT and Sp on Bax was not significant (P-Value = 0.48, F = 0.51 and Eta: 0.02) (Figure 1).
No significant difference was observed in BCL-2 levels in HC and HFD groups (P-Value = 0.48). However, BCL-2 levels in the HFD+Sp (P-Value = 0.008), HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups were higher than the HFD group. Also, BCL-2 levels in the HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups were significantly higher than the HFD+Sp group as well as in the HFD+HIIT+Sp group were significantly higher than the HFD+HIIT group (P-Value = 0.001) (Figure 2). Also, HIIT (P-Value = 0.001, F = 258.39 and Eta: 0.92) and Sp (P-Value = 0.001, F=63.19 and Eta: 0.76) had a significant effect on increasing BCL-2 gene expression in the hippocampal tissue of HFD-induced obese rats. Moreover, the interaction of HIIT and Sp on the increase of BCL-2 was significant (P-Value = 0.006, F = 9.59 and Eta: 0.32) (Figure 2).
Mean and standard deviation were used for descriptive statistics. Shapiro-Wilk test was used to check the normality of data distribution. Next, according to the normality of the data distribution, one-way analysis of variance (ANOVA) along with Tukey's post-hoc tests and two-way ANOVA along with Bonferonie's post-hoc tests were used for analyzing data in the SPSS software version 20. A significance level of 0.05 was considered for all analyses.
Results
The results of one-way ANOVA test showed a significant difference in the gene expression levels of Bax (F = 23.31 and P-Value = 0.001), BCL-2 (F = 115.83 and P-Value = 0.001) and Bax/BCL-2 (F = 313.22 and P-Value = 0.001) in research groups. The results of Tukey's post-hoc test showed that the Bax levels in the HFD group were significantly higher than the HC group (P-Value = 0.016). No significant difference was observed in the HFD and HFD+Sp groups (P-Value = 0.34); however, in the HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups, Bax levels were significantly lower than the HFD group, also in the HFD+HIIT (P-Value = 0.006) and HFD+HIIT+Sp (P-Value = 0.001) groups were significantly lower than the HFD+Sp group as well as in the HFD+HIIT+Sp group were significantly lower than the HFD+HIIT group (P-Value = 0.04). The results of the two-way ANOVA showed that the HIIT (P-Value = 0.001, F = 68.05 and Eta: 0.77) and Sp (P-Value = 0.004, F = 10.27 and Eta: 0.33) had a significant effect on the reduction of Bax gene expression in the hippocampal tissue of HFD-induced obese rats. Nevertheless, the interaction of HIIT and Sp on Bax was not significant (P-Value = 0.48, F = 0.51 and Eta: 0.02) (Figure 1).
No significant difference was observed in BCL-2 levels in HC and HFD groups (P-Value = 0.48). However, BCL-2 levels in the HFD+Sp (P-Value = 0.008), HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups were higher than the HFD group. Also, BCL-2 levels in the HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups were significantly higher than the HFD+Sp group as well as in the HFD+HIIT+Sp group were significantly higher than the HFD+HIIT group (P-Value = 0.001) (Figure 2). Also, HIIT (P-Value = 0.001, F = 258.39 and Eta: 0.92) and Sp (P-Value = 0.001, F=63.19 and Eta: 0.76) had a significant effect on increasing BCL-2 gene expression in the hippocampal tissue of HFD-induced obese rats. Moreover, the interaction of HIIT and Sp on the increase of BCL-2 was significant (P-Value = 0.006, F = 9.59 and Eta: 0.32) (Figure 2).
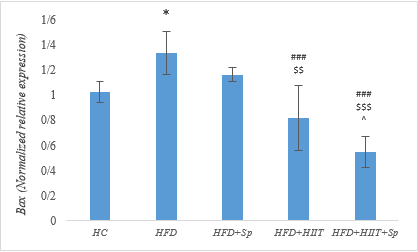 Figure 1. Bax gene expression levels in the brain tissue of rats in research groups. * (P-Value ≤ 0.05): significant increase compared to HC group, ### (P-Value ≤ 0.001): significant decrease compared to HFD group, $$ (P-Value ≤ 0.01) and $$$ (P-Value ≤ 0.001): significant decrease compared to the HFD+Sp group; ^ (P-Value ≤ 0.05): significant decrease compared to HFD+HIIT group. 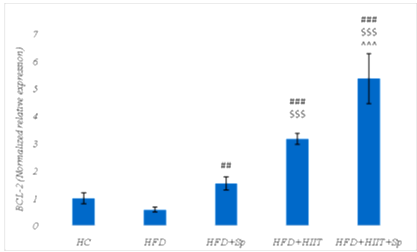 Figure 2. BCL-2 gene expression levels in the brain tissue of rats in the research groups. ## (P-Value ≤ 0.01) and ### (P-Value ≤ 0.001): significant increase compared to HFD group, $$$ (P-Value ≤ 0.001): significant increase compared to HFD+Sp group; ^^^ (P-Value ≤ 0.001): significant increase compared to the HFD+HIIT group. |
The ratio of Bax to BCL-2 levels in the HFD group was significantly higher than HC group (P-Value = 0.001). However, BCL-2 levels in HFD+Sp (P-Value = 0.008), HFD+HIIT (P-Value = 0.001), and HFD+HIIT+Sp (P-Value = 0.001) groups were lower than in the HFD group. Also, BCL-2 levels in the HFD+HIIT (P-Value = 0.001) and HFD+HIIT+Sp (P-Value = 0.001) groups were significantly lower than HFD+Sp group. No significant difference was observed between HFD+HIIT+Sp and HFD+HIIT groups (P-Value = 0.22). Also, HIIT (P-Value = 0.001, F=632.69 and effect size 0.96) and Sp (P-Value = 0.001, F=248.002 and Eta: 0.92) had a significant effect on reducing Bax/BCL-2 in the hippocampal tissue of HFD-induced obese rats. Also, the interactive effect of HIIT and Sp on the reduction of Bax/BCL-2 was significant (P-Value = 0.001, F = 167.41 and Eta: 0.89) (Figure 3).
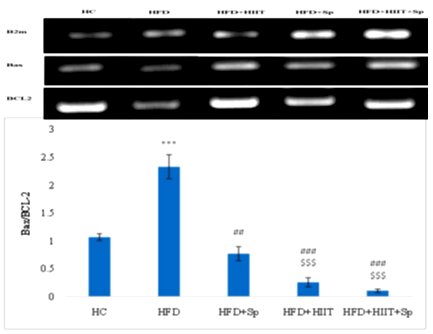 Figure 3. Bax/BCL-2 gene expression levels in the brain tissue of rats in the research groups. *** (P-Value ≤0.001): significant increase compared to HC group; ## (P-Value ≤0.01) and ### (P-Value ≤0.001): significant decrease compared to HFD group, $$$ (P-Value ≤0.001): significant decrease compared to HFD + Sp group. |
Discussion
The hypothesis of this study was whether HIIT and Sp have a significant effect on the changes of Bax, BCL-2 gene expression and the Bax/BCL-2 in the hippocampal tissue of HFD-induced obese rats. The results showed that Bax levels and Bax/BCL-2 ratio were significantly higher in the HFD group than in the HC group. The research has shown that increasing obesity may increase the risk of Alzheimer's disease by 36%. It seems that obesity and increased fat mass are related to the decrease in the volume of gray matter in the brain, disorders in the temporal, insular, frontal cortex and other parts of the brain. In addition, the increase of ROS can lead to disruption of the immune system by reducing the power of mitochondrial biogenesis, and increasing inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in the nervous system (20). Therefore, conducting basic studies to find solutions for inhibiting inflammation, apoptosis, and cognitive impairment in obese individuals is essential.
However, the results of the present study showed that Bax levels and Bax/BCL-2 ratios in the HFD+HIIT group were significantly lower and the BCL-2 levels were significantly higher than in the HFD group. Studies show that exercises with the mechanism of increasing neurogenesis, reducing the loss of dopaminergic neurons, increasing cerebral blood flow, increasing antioxidant capacity and improving autophagy lead to improved synapse function. In other words, long-term exercises increase phosphatidyl-inositol-3 kinase (PI3K) and nuclear respiratory transcription factor 2 (NRF2), leading to increased transcription of SOD and Cat. Furthermore, long-term exercises increase neurotrophins, such as BDNF and nerve growth factor (NGF), resulting in the inhibition of procaspase-9, caspase 8, and caspase 3, and this further leads to the inhibition of tumor necrosis factor-alpha family-related ligand FASL. Inhibiting FASL prevents the release of cytochrome C, which, in turn, promotes increased expression of BCL-2 and BCL-XL as a downstream effect of these exercises. As a result, the increase in these anti-apoptotic proteins leads to a decrease in the apoptotic proteins Bax and BAK, and the Bax/BCL-2 ratio in the brain tissue (21). In addition, researchers have shown that activated PI3K after exercise leads to the activation of CREB nuclear protein, and this leads to the activation of the PI3K/AKT anti-apoptotic pathway and BDNF, pTrkB, pGRB2, and GAB1 proteins, pPI3K, and pAKT increase and this leads to the activation of the MEK/MAPK/ERK cascade (22). In this context, a study showed that aerobic training led to a decrease in the expression of death-associated protein kinase 1 (DAPK1) and FASL in the brain tissue of aged rats (23). Also, in a study, four weeks of aerobic training led to a decrease in the expression of caspase-3, MDA, and Bax, while increased BCL-2, BDNF, SOD, and Cat in the brain tissue of rats exposed to deep-heated oil (6). In another study, endurance training led to the inhibition of apoptotic proteins and the increase of anti-apoptotic proteins in the cortex tissue of diabetic rats (22). Also, in a study, exercise led to a reduction of inflammatory factors such as TNF-α, resistin, and free fatty acids in the hippocampal tissue of animals fed a high-fat diet (24). In another study, researchers compared two types of HIIT with 4-minute and 1-minute intervals and their results showed that although some anti-apoptotic adaptations in the brain tissue are dependent on the intervals, both methods of resistance training had a favorable effect on increasing the anti-apoptotic markers in the brain tissue of rats suffering from stroke (25). Therefore, from the anti-apoptotic point of view, our study confirmed the results of previous studies. Therefore, it seems that performing exercises, especially with the current research protocol, may inhibit neuronal apoptosis and consequently reduce the risk of cognitive disorders in obesity.
The results of the present study showed that in the HFD+Sp group, BCL-2 levels were significantly higher and Bax/BCL-2 levels were significantly lower than the HFD group. It seems that the main ingredient in Sp is phycocyanin-C, which can combine with peroxyl and hydroxyl free radicals in the brain tissue as soon as possible, prevent the activity of lipid peroxidation, and ultimately lead to the modulation of the immune system in different parts of the brain (26). For example, in a study, researchers showed that Sp, in addition to having non-enzymatic antioxidants such as vitamin B6 and B12, with the mechanism of reducing free radicals, can lead to the inhibition of inflammatory factors and the reduction of TNF-α, 6-hydroxydopamine (6-OHDA) and MDA, as well as increases SOD, GPx, nitric oxide (NO), thereby improving antioxidant capacity (27). In another study, it was stated that Sp, by reducing lipopolysaccharides, leads to a decrease in inflammation, an increase in the expression of BDNF, activation of phosphorylated protein kinase A (pAKT), a decrease in cooxygenase-2 (COX-2), an increase in NRF2, p-cAMP, and p-CREB leads to the transcription of metabolic genes and increasing the expression of BCL-2 in brain tissue (28). In this context, researchers found that 500 mg/kg of Sp for 14 days led to an increase in SOD, GPx, Ca, and a decrease in IL-1 and IL-6 in the brain tissue of rats exposed to methotrexate (29).
The results showed that in the HFD+HIIT+Sp group, Bax and Bax/BCL-2 levels were significantly lower, whereas BCL-2 levels were significantly higher than the HFD group. In the context of the simultaneous effect of exercise and Sp on brain tissue, a study showed that six weeks of resistance training and Sp simultaneously improved antioxidants, reduced oxidative stress, improved cognitive function, and increased SOD in the hippocampal tissue of rats exposed to stanozolol (14). It seems that exercise with the mechanism of activation of PI3K/NRF2 signaling, increase of expression of SOD and Cat, increase of BDNF and NGF, activation of PI3K/AKT/CEREB signaling, leads to inhibition of procaspase-9, caspase 8, caspase 3, FASL, decrease of BAX, BAK, release of cytochrome C, increase the expression of BCL-2 and BCL-XL (21,22). Also, Sp with the mechanism of neutralizing free radicals, reducing lipid peroxidation (26), inhibition of TNF-α, 6-OHDA, and MDA, increase of SOD, GPx, and NO (Saraswathi, 2023), increase of BDNF, activation of pAKT, decrease of COX-2, increase of NRF2, p-cAMP, and p-CREB, leads to the transcription of metabolic genes, increasing the expression of BCL-2 and decreasing Bax (27). Therefore, the information shows that exercise and Sp supplement are effective in improving neuronal function and reducing apoptosis in brain tissue. Since Bax and Bax/BCL-2 levels were significantly lower and BCL-2 levels significantly higher in the HFD+HIIT and HFD+HIIT+Sp groups compared to the HFD+Sp group. Exercises that more effectively target lipid metabolism through catecholamine pathways and enhance cAMP phosphorylation appear to have a stronger impact than Sp (16). In this regard, the results of a study showed that the combination of aerobic exercise and Sp on increasing nesfatin 1 and neuropeptide gamma was more favorable than the effect of Sp (16). Also, according to the results of the present study, Bax levels were significantly lower, and BCL-2 levels were significantly higher in the HFD+HIIT+Sp group than HFD+HIIT group. It seems that exercise and Sp supplement probably strengthen each other's effect and have a synergistic effect in reducing neuronal apoptosis through common pathways. Consistent with our findings, Aghahi et al. demonstrated that the combined effect of exercise and Sp more effectively reduced miR125b and miR146a (Markers of oxidative stress) and improved cognitive function compared to either intervention alone (14). In addition to examining the effects of the combination of exercise and Sp in human samples, a study also showed that HIIT with Sp led to an increase in VO2 peak, omentin-1, irisin, spexin and a decrease in lipocalin-2 and asprosin and improved the body composition of obese men (30). In another study by the same researchers, the combination of HIIT and Sp training led to the improvement of fat profile, reduction of TNF-α, C-reactive protein (CRP), Sema3C, IL-6, monocyte protein-1 (MCP-1) and IL-8 in obese men (30). Also, in a study, researchers showed that aerobic exercise and Sp simultaneously increased Nesfatin-1 and Peptide YY in overweight elderly men (16). Therefore, it seems that the combination of these two interventions, both clinically and in experimental studies on animals, can improve metabolic and nervous system disorders.
Considering the role of oxidative stress and antioxidants in the promotion of apoptosis, the lack of measurement of these variables was one of the limitations of the present study. Therefore, it is suggested to evaluate oxidant-antioxidant markers in future studies. Also, considering the role of neurotrophins in inhibiting apoptosis, which was not measured in the present study, it is suggested to evaluate neurotrophins in future studies. In addition, it seems that the inability to generalize the results to human samples, different methods of measuring variables and considering different exercise intensities are other limitations of the present study. Therefore, it is suggested that these limitations be used as research manipulations in future studies.
Conclusion
It seems that HIIT and Sp supplement separately have a beneficial effect on some apoptotic markers in hippocampal tissue in obese rats. However, their interactive effect on inhibiting neuronal apoptosis in brain tissue in obesity conditions is more obvious than the effect of each one alone.
Acknowledgement
This manuscript is a part of a doctoral degree by Soraya Pourasghari Siah Astalkhi, a doctoral student at Azad University of North Tehran. The research assistant of this academic unit is hereby appreciated. We also acknowledge the animal laboratory expert of Islamic Azad University, Marvdasht branch, Omidreza Salehi, for his guidance and help.
Funding sources
The authors received no financial support from any organization.
Ethical statement
All the ethical principles of working with animals in this research were carried out under the supervision of the Ethics Committee in Biomedical Research of the Islamic Azad University, Sanandaj branch, with the approval no. IR.IAU.SDJ.REC.1401.099.
Conflicts of interest
The authors declared no conflicts of interest.
Author contributions
In this manuscript, Soraya Pourasghari Siah Astalkhi has been responsible for financial support, data preparation, and practical research protocol. The initial writing of the manuscript was done by Seyed Morteza Tayibi, Soraya Pourasghari Siah Astalkhi, and Rasul Islami. The research implementation was supervised by Seyed Morteza Tayibi and Lida Moradi. Seyed Morteza Tayibi was also the final editor of the manuscript.
Type of Article: Original article |
Subject:
General medicine
Received: 2024/07/7 | Accepted: 2024/09/3 | Published: 2025/05/21
Received: 2024/07/7 | Accepted: 2024/09/3 | Published: 2025/05/21
References
1. Kim C, Fryar C, Ogden CL. Epidemiology of obesity. In: Handbook of epidemiology. New York:Springer;2023. p.1-47 [View at Publisher] [DOI] [Google Scholar]
2. Hosseini SA, Norouzi S, Rafiee N, Farzanegi P, Salehi O, Farkhaie F. Interactive Effects of Endurance Training and Crocin on Aerobic Capacity, Dietary Intake and Weight of High-Fat Diet-Induced Type 2 Diabetic Rats. J Nutr Sci Diet. 2018;4(3):65-74. [View at Publisher] [Google Scholar]
3. Hosseini SA, Hamzavi K, Safarzadeh H, Salehi O. Interactive effect of swimming training and fenugreek (Trigonella foenum graecum L.) extract on glycemic indices and lipid profile in diabetic rats. Arch Physiol Biochem. 2023;129(2):349-53. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Li H, Ren J, Li Y, Wu Q, Wei J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol (Lausanne). 2023;14:1134025. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Naomi R, Teoh SH, Embong H, Balan SS, Othman F, Bahari H, et al. The Role of Oxidative Stress and Inflammation in Obesity and Its Impact on Cognitive Impairments-A Narrative Review. Antioxidants (Basel). 2023;12(5):1071. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Nikbin S, Fardad G, Yazdi S, Bahman MH, Ettefagh P, Khalegi F, et al. RETRACTED: Aerobic exercise training reduces deep-frying oil-induced apoptosis of hippocampal tissue by reducing oxidative stress in male rats. J Chem Neuroanat. 2023;133:102328. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Muscella A, Stefàno E, Marsigliante S. The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Circ Physiol. 2020;319(1):H76-88. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. de Souza RF, de Moraes SRA, Augusto RL, de Freitas Zanona A, Matos D, Aidar FJ, et al. Endurance training on rodent brain antioxidant capacity: A meta-analysis. Neurosci Res. 2019;145:1-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Camiletti‐Moirón D, Aparicio VA, Aranda P, Radak Z. Does exercise reduce brain oxidative stress? A systematic review. Scand J Med Sci Sports. 2013;23(4):e202-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Ni C, Ji Y, Hu K, Xing K, Xu Y, Gao Y. Effect of exercise and antioxidant supplementation on cellular lipid peroxidation in elderly individuals: Systematic review and network meta-analysis. Front Physiol. 2023;14:01-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Ghanbari P, Khajehzadeh S, Sayyed A, Raeisi D, Salehi O. The effect of high intensity interval training with beetroot (Beta vulgaris) juice supplementation on serotonin and dopamine receptors expression, anxiety and depression in middle-aged diabetic rats. Avicenna J Phytomed. 2022;12(6):627-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Bohórquez-Medina SL, Bohórquez-Medina AL, Zapata VAB, Ignacio-Cconchoy FL, Toro-Huamanchumo CJ, Bendezu-Quispe G, et al. Impact of spirulina supplementation on obesity-related metabolic disorders: A systematic review and meta-analysis of randomized controlled trials. NFS J. 2021;25:21-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Trotta T, Porro C, Cianciulli A, Panaro MA. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients. 2022;14(3):676. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Agahi MRH, Mosallanejad Z, Salehi OR. The effects of resistance training and spirulina on the performance of the antioxidant system with emphasis on mir125b, mir146a and cognitive function in stanazolol-induced neurotoxicity in rats. Chem Biol Interact. 2022;366:110112. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Abouzed TK, Soliman MM, Khatab SA, Gouda WM, Eldomany EB, Dorghamm DA. The protective impacts of Spirulina platensis against cisplatin-induced renal injury through the regulation of oxidative stress, pro-inflammatory cytokines and Bax/Bcl2. Toxicol Res (Camb). 2022;11(1):169-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Babaei M, Abdi A, Mehrabani J, Daloii AA. The Effect of Aerobic Training and Spirulina on Nesfatin-1 and Peptide YY in Overweight Elderly Men: A randomized trial. Iran J Heal Sci. 2022; [View at Publisher] [DOI] [Google Scholar]
17. Gajda AM. High fat diets for diet-induced obesity models. Res Diets. 2008;8:1-3. [View at Publisher] [Google Scholar]
18. Azhdari A, Hosseini SA, Farsi S. Antioxidant effect of high intensity interval training on cadmium-induced cardiotoxicity in rats. Gene, Cell Tissue. 2019;6(3):e94671. [View at Publisher] [DOI] [Google Scholar]
19. Gad AS, Khadrawy YA, El-Nekeety AA, Mohamed SR, Hassan NS, Abdel-Wahhab MA. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. 2011;27(5):582-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Schmitt LO, Gaspar JM. Obesity-induced brain neuroinflammatory and mitochondrial changes. Metabolites. 2023;13(1):86. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Andreotti DZ, Silva J do N, Matumoto AM, Orellana AM, De Mello PS, Kawamoto EM. Effects of physical exercise on autophagy and apoptosis in aged brain: Human and animal studies. Front Nutr. 2020;7:94. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Cheng SM, Lee SD. Exercise training enhances BDNF/TrkB signaling pathway and inhibits apoptosis in diabetic cerebral cortex. Int J Mol Sci. 2022;23(12):6740. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Liu Y, Guo W, Hong SL. Aerobic exercise mitigates hippocampal neuronal apoptosis by regulating DAPK1/CDKN2A/REDD1/FoXO1/FasL signaling pathway in D‐galactose‐induced aging mice. FASEB J. 2023;37(10):e23205. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Della Guardia L, Codella R. Exercise restores hypothalamic health in obesity by reshaping the inflammatory network. Antioxidants (Basel). 2023;12(2):297. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Hugues N, Pin-Barre C, Brioche T, Pellegrino C, Berton E, Rivera C, et al. High-intensity training with short and long intervals regulate cortical neurotrophic factors, apoptosis markers and chloride homeostasis in rats with stroke. Physiol Behav. 2023;266:114190. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Almeida T, Manfroi G, Silva S, Beggiora P, Schwingel D, Bertolin TE. Exploring the Neuroprotective Effects of Spirulina platensis: Insights Into Hemorrhagic Volume and Histological Outcomes. Cureus. 2023;15(7):e42078. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Saraswathi K, Kavitha CHN. Spirulina: Pharmacological Activities and Health Benefits. J Young Pharm. 2023;15(3):441-7. [View at Publisher] [DOI] [Google Scholar]
28. Sorrenti V, Castagna DA, Fortinguerra S, Buriani A, Scapagnini G, Willcox DC. Spirulina microalgae and brain health: A scoping review of experimental and clinical evidence. Mar Drugs. 2021;19(6):293. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Behairy A, Elkomy A, Elsayed F, Gaballa MMS, Soliman A, Aboubakr M. Antioxidant and anti-inflammatory potential of spirulina and thymoquinone mitigate the methotrexate-induced neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(3):1875-88. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Supriya R, Delfan M, Saeidi A, Samaie SS, Al Kiyumi MH, Escobar KA, et al. Spirulina Supplementation with High-Intensity Interval Training Decreases Adipokines Levels and Cardiovascular Risk Factors in Men with Obesity. Nutrients. 2023;15(23):4891. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |