Volume 10, Issue 1 (3-2022)
Jorjani Biomed J 2022, 10(1): 56-66 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Alemrajabi M, Sadrzadeh-Afshar M, dastorani M, Barjestehnia M. Effects of Propolis and Persica Mouthwashes on Minor Aphthous Ulcers: A Comparative Study. Jorjani Biomed J 2022; 10 (1) :56-66
URL: http://goums.ac.ir/jorjanijournal/article-1-872-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-872-en.html
Mohammad-Sadegh Alemrajabi1 

 , Maryam-Sadat Sadrzadeh-Afshar
, Maryam-Sadat Sadrzadeh-Afshar 

 2, Mahdi Dastorani3
2, Mahdi Dastorani3 

 , Meysam Barjestehnia4
, Meysam Barjestehnia4 




 , Maryam-Sadat Sadrzadeh-Afshar
, Maryam-Sadat Sadrzadeh-Afshar 

 2, Mahdi Dastorani3
2, Mahdi Dastorani3 

 , Meysam Barjestehnia4
, Meysam Barjestehnia4 


1- Assistant Professor, Oral & Maxillofacial medicine department, Faculty of Dentistry, Aja University of medical sciences, Tehran, Iran
2- Assistant professor, Oral and maxilofacial medicine department, Faculty of Dentistry, Aja university of medical sciences, Tehran, Iran , m_sadrzade@alumnus.tums.ac.ir
3- Assistant Professor ,Endodontics department, Faculty of Dentistry, Aja University of medical sciences, Tehran, Iran
4- Dentist, Oral & Maxillofacial medicine department, Faculty of Dentistry, Aja University of medical sciences, Tehran, Iran
2- Assistant professor, Oral and maxilofacial medicine department, Faculty of Dentistry, Aja university of medical sciences, Tehran, Iran , m_sadrzade@alumnus.tums.ac.ir
3- Assistant Professor ,Endodontics department, Faculty of Dentistry, Aja University of medical sciences, Tehran, Iran
4- Dentist, Oral & Maxillofacial medicine department, Faculty of Dentistry, Aja University of medical sciences, Tehran, Iran
Full-Text [PDF 481 kb]
(679 Downloads)
| Abstract (HTML) (2455 Views)
.png)
.png)
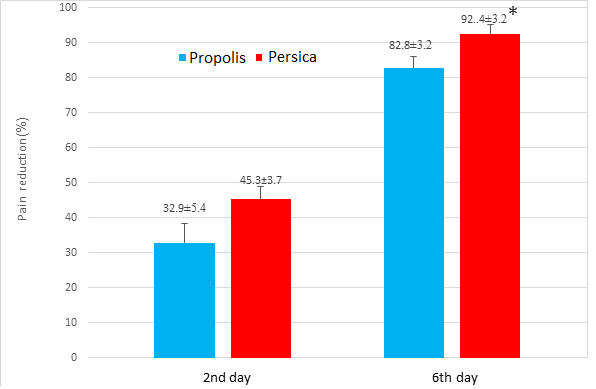
The mean percentage of pain intensity reduction on the second day of treatment was not significantly different between the two groups of propolis (32.9± 0.54) and Persica (45.3±3.7) (P=0.07), but the percentage of pain intensity reduction on the sixth day of treatment in the Persica (92.4± 3.2) group was significantly higher than in the propolis group (82.8±3.2) (P=0.03) (Figure 2 & Chart 1)
The mean percentage of ulcer size reduction on the second day of treatment was not significantly different between propolis (22.9±5.5) and Persica (34.1±3.8) groups (P=0.10), but the percentage of ulcer size reduction on the sixth day of treatment in Persica (95.0±2.4) group was significantly higher than propolis group (76.0±8.9) (P=0.05), as seen in Table 2 & Chart 2. Data were reported as mean ± SEM and analyzed statistically by Man-Whitney test at a significance level (*) of P<0.05.
Aphthous stomatitis is a multifactorial disease, the exact cause has not yet been determined.
Many drugs are currently used to treat aphthous stomatitis, most of which are chemical and have side effects. Therefore herbal medicine can be promising.
Full-Text: (135 Views)
Introduction
Recurrent Aphthous Stomatitis (RAS) is a common disease that affects about 20% of the general population (1), and is a nonkeratinized oral mucosal disorder characterized by painful ulcerations and inflammation, causing difficulty in eating, swallowing, and speaking. The clinical symptoms of the disorder typically include irritation, itching, and local pain for 24-48 hours after the onset of ulcer formation. Aphthous lesions are distinctly round superficial ulcer with a necrotic center surrounded by erythematous haloes. Pain intensity decreases with re-epithelialization after four days (1, 3). The prevalence of RAS is slightly higher in women, and the duration of the ulcer is usually 7-10 days. Eighty percent of RAS cases occur before the age of 30 years (1-4).
Aphthous stomatitis is a multifactorial disease, which may be caused by trauma, infections, deficiency of folic acid and B-complex vitamins, immune factors, psychological stress and food allergens. Most research to date has focused on immune factors. However, the exact cause of RAS has not yet been determined and its treatment is challenging. Most treatments currently focus on symptomatic therapies, including analgesics, anesthetics, antiseptics, anti-inflammatory drugs, steroids, and sucralfate (5, 6).
Possible therapeutic goals for aphthous stomatitis includes alleviating the symptoms, reducing the number and size of ulcers and shortening the duration of disease (1, 3). Various clinical trials have been performed to treat RAS. Their results indicate the effect of chlorhexidine in reducing the severity and duration of the disease, but not the frequency of ulcers, indicating the presence of an infectious agent for the cause of RAS. In addition, topical steroids have been shown to reduce the severity and duration of ulcers, indicating the involvement of the immune system in the development of aphthous stomatitis (1, 7). The effectiveness of any of these drugs has not been proven in all patients with RAS.
Persica is an herbal mouthwash containing the active ingredients of toothbrush tree, menthe, and yarrow. The most important ingredients are tannins, essential oils, calcium and chloride. Unlike other mouthwash solutions, this solution does not cause any side effects if swallowed and its use is unobstructed in children and pregnant women. The chemical compounds of Persica mouthwash include sodium chloride, silica, sulfur, fluorine, trimethylamine, vitamin C and resin, which together cause antimicrobial, antifungal, anti-plaque, and anti-caries properties of this plant (8). Previous clinical trials have shown that continuous use of the toothbrush tree due to the high level of chloride reduces dental plaque formation, prevents tooth discoloration and gingivitis, and the isothiocyanate compounds of this plant inhibit the growth of oral bacteria. In addition, high levels of available calcium increase mineralization and tooth strength (9-11).
Propolis is a Greek word that means to defend the city, and bees use it for purposes such as patching a hole in a hive, flattening the inner walls of the hive, and Mummification the bodies of insects trapped inside the hive and dead, and the bees are unable to get them out. In addition, propolis protects their colonies from diseases due to antiseptic effect and antimicrobial properties. Propolis is one of the most important bee products which is effective on bacterial growth and enzyme inhibition through inhibition of cell division and protein synthesis, and its effect on the bacterial cytoplasm. Therefore, propolis acts as a natural antibiotic and has no side effects compared to industrial antibiotics, so it can be considered as a suitable alternative. Flavonoids make up the bulk of the propolis resin and are responsible for most of the antioxidant, antibacterial, antiviral, antifungal, anticancer, and anti-inflammatory properties of propolis. Ethanolic extract of propolis exerts anti-inflammatory and analgesic effects in rat models by inhibiting nitric oxide production. The results showed that even these anti-inflammatory and analgesic effects are equivalent to one 20 mg diclofenac tablet (12). Studies show that propolis has an inhibitory effect on various bacterial strains, some fungal species and protozoa, and a wide range of viruses (11, 13-15). According to the properties mentioned about propolis and persica, the aim of this study was to evaluate and compare the effect of propolis and persica mouthwashes in the treatment of minor aphthous ulcers and reduce their complications including pain and burning.
Materials and Methods
The current study was conducted on 40 patients including 26 males and 14 females aged between 18-56 years. Inclusion criteria were complaining of minor aphthous ulcers on the first day of appearing of ulcers which were approved by an alert clinician based on clinical examination. Exclusion criteria were any history of systemic disease including Bechet syndrome, Granulomatous diseases such as crohn disease and sarcoidosis, Celiac disease and HIV infection. Also, patients with disease or conditions affecting the healing process such as diabetes mellitus, metabolic diseases, any suppression of the immune system, smoking and alcohol consumers were not enrolled in the study (16, 17). The patients were divided into two groups of 20 via systematic random sampling. This study was single-blinded and patients and clinicians were unaware of type of the mouthwashes. Group A received Persica mouthwash (Poursina Pharmaceutical Co., Iran), the group B received propolis mouthwash containing 30% extract (Soren Tech Toos Company, Iran). The method of using mouthwashes was the same and the dosage for the treatment of ulcers was three times a day and each time 15 drops in 15 ml of water (three tablespoons), which was circulated in the mouth for 20 seconds and then discarded according to the manufactures instruction. The patient was asked to use this mouthwash in the same way from the first day of appearing ulcers for 10 days. The intensity of pain and burning was recorded by measuring the score of Visual Analogue Scale (VAS) (18) and the diameter of minor aphthous lesions (in mm) was recorded using a periodontal probe at three periods before treatment, and on the second and sixth days after treatment. The intensity of pain and burning and the indicators set for healing time were assessed and recorded on days 0 (before treatment), 2 and 6 of treatment, and compared with the pre-treatment scales recorded for the two types of mouthwash (3). Changes in pain intensity and ulcer size scores on the second and sixth days of treatment were calculated as follows. The values of the second or sixth day of treatment were subtracted from the pre-treatment values and divided by the pre-treatment values and finally multiplied by 100 to calculate the percentage of changes (3, 19).
Statistical analysis
The data were analyzed in SPSS 22 software by Man-Whitney test, and P<0.05 was considered a significant difference.
Results
There was no significant difference (P=0.16) in the mean pain intensity score before treatment between the two groups receiving propolis (4.0 ± 3.7) and Persica (3.0 ± 1.8) (Figure 1). There was no significant difference in the mean pain intensity score on the second day of treatment between the two groups receiving propolis (5.0 ± 0.5) and Persica (3.0 ± 4.4) according to P-Value (P=0.29) but the pain intensity on the sixth day of treatment in the Persica group (2.0 ± 6.0) was significantly lower than in the propolis group (3.0 ± 4.1) (P=0.05). There was no significant difference in the mean ulcer size before treatment between propolis (2.0 ± 1.3 mm) and persica groups (3.5±0.2mm) (P=0.11), indicating the homogeneity of the two groups (Figure 2). There was no significant difference in the mean ulcer size on the second (P=0.97) day of treatment between the two groups of propolis (2.3 ±0.2 mm) and Persica (2.3 ±0.2 mm). There was no significant difference in the mean ulcer size on the sixth (P=0.10) day of treatment between the two groups of propolis (0.8± 0.3 mm) and Persica (2.0 ± 6.0 mm). The mean duration of ulcer healing in the Persica (5.2± 0.2) group was significantly shorter than the propolis group (0.6± 0.3) (P=0.03) (Table1).
Aphthous stomatitis is a multifactorial disease, which may be caused by trauma, infections, deficiency of folic acid and B-complex vitamins, immune factors, psychological stress and food allergens. Most research to date has focused on immune factors. However, the exact cause of RAS has not yet been determined and its treatment is challenging. Most treatments currently focus on symptomatic therapies, including analgesics, anesthetics, antiseptics, anti-inflammatory drugs, steroids, and sucralfate (5, 6).
Possible therapeutic goals for aphthous stomatitis includes alleviating the symptoms, reducing the number and size of ulcers and shortening the duration of disease (1, 3). Various clinical trials have been performed to treat RAS. Their results indicate the effect of chlorhexidine in reducing the severity and duration of the disease, but not the frequency of ulcers, indicating the presence of an infectious agent for the cause of RAS. In addition, topical steroids have been shown to reduce the severity and duration of ulcers, indicating the involvement of the immune system in the development of aphthous stomatitis (1, 7). The effectiveness of any of these drugs has not been proven in all patients with RAS.
Persica is an herbal mouthwash containing the active ingredients of toothbrush tree, menthe, and yarrow. The most important ingredients are tannins, essential oils, calcium and chloride. Unlike other mouthwash solutions, this solution does not cause any side effects if swallowed and its use is unobstructed in children and pregnant women. The chemical compounds of Persica mouthwash include sodium chloride, silica, sulfur, fluorine, trimethylamine, vitamin C and resin, which together cause antimicrobial, antifungal, anti-plaque, and anti-caries properties of this plant (8). Previous clinical trials have shown that continuous use of the toothbrush tree due to the high level of chloride reduces dental plaque formation, prevents tooth discoloration and gingivitis, and the isothiocyanate compounds of this plant inhibit the growth of oral bacteria. In addition, high levels of available calcium increase mineralization and tooth strength (9-11).
Propolis is a Greek word that means to defend the city, and bees use it for purposes such as patching a hole in a hive, flattening the inner walls of the hive, and Mummification the bodies of insects trapped inside the hive and dead, and the bees are unable to get them out. In addition, propolis protects their colonies from diseases due to antiseptic effect and antimicrobial properties. Propolis is one of the most important bee products which is effective on bacterial growth and enzyme inhibition through inhibition of cell division and protein synthesis, and its effect on the bacterial cytoplasm. Therefore, propolis acts as a natural antibiotic and has no side effects compared to industrial antibiotics, so it can be considered as a suitable alternative. Flavonoids make up the bulk of the propolis resin and are responsible for most of the antioxidant, antibacterial, antiviral, antifungal, anticancer, and anti-inflammatory properties of propolis. Ethanolic extract of propolis exerts anti-inflammatory and analgesic effects in rat models by inhibiting nitric oxide production. The results showed that even these anti-inflammatory and analgesic effects are equivalent to one 20 mg diclofenac tablet (12). Studies show that propolis has an inhibitory effect on various bacterial strains, some fungal species and protozoa, and a wide range of viruses (11, 13-15). According to the properties mentioned about propolis and persica, the aim of this study was to evaluate and compare the effect of propolis and persica mouthwashes in the treatment of minor aphthous ulcers and reduce their complications including pain and burning.
Materials and Methods
The current study was conducted on 40 patients including 26 males and 14 females aged between 18-56 years. Inclusion criteria were complaining of minor aphthous ulcers on the first day of appearing of ulcers which were approved by an alert clinician based on clinical examination. Exclusion criteria were any history of systemic disease including Bechet syndrome, Granulomatous diseases such as crohn disease and sarcoidosis, Celiac disease and HIV infection. Also, patients with disease or conditions affecting the healing process such as diabetes mellitus, metabolic diseases, any suppression of the immune system, smoking and alcohol consumers were not enrolled in the study (16, 17). The patients were divided into two groups of 20 via systematic random sampling. This study was single-blinded and patients and clinicians were unaware of type of the mouthwashes. Group A received Persica mouthwash (Poursina Pharmaceutical Co., Iran), the group B received propolis mouthwash containing 30% extract (Soren Tech Toos Company, Iran). The method of using mouthwashes was the same and the dosage for the treatment of ulcers was three times a day and each time 15 drops in 15 ml of water (three tablespoons), which was circulated in the mouth for 20 seconds and then discarded according to the manufactures instruction. The patient was asked to use this mouthwash in the same way from the first day of appearing ulcers for 10 days. The intensity of pain and burning was recorded by measuring the score of Visual Analogue Scale (VAS) (18) and the diameter of minor aphthous lesions (in mm) was recorded using a periodontal probe at three periods before treatment, and on the second and sixth days after treatment. The intensity of pain and burning and the indicators set for healing time were assessed and recorded on days 0 (before treatment), 2 and 6 of treatment, and compared with the pre-treatment scales recorded for the two types of mouthwash (3). Changes in pain intensity and ulcer size scores on the second and sixth days of treatment were calculated as follows. The values of the second or sixth day of treatment were subtracted from the pre-treatment values and divided by the pre-treatment values and finally multiplied by 100 to calculate the percentage of changes (3, 19).
Statistical analysis
The data were analyzed in SPSS 22 software by Man-Whitney test, and P<0.05 was considered a significant difference.
Results
There was no significant difference (P=0.16) in the mean pain intensity score before treatment between the two groups receiving propolis (4.0 ± 3.7) and Persica (3.0 ± 1.8) (Figure 1). There was no significant difference in the mean pain intensity score on the second day of treatment between the two groups receiving propolis (5.0 ± 0.5) and Persica (3.0 ± 4.4) according to P-Value (P=0.29) but the pain intensity on the sixth day of treatment in the Persica group (2.0 ± 6.0) was significantly lower than in the propolis group (3.0 ± 4.1) (P=0.05). There was no significant difference in the mean ulcer size before treatment between propolis (2.0 ± 1.3 mm) and persica groups (3.5±0.2mm) (P=0.11), indicating the homogeneity of the two groups (Figure 2). There was no significant difference in the mean ulcer size on the second (P=0.97) day of treatment between the two groups of propolis (2.3 ±0.2 mm) and Persica (2.3 ±0.2 mm). There was no significant difference in the mean ulcer size on the sixth (P=0.10) day of treatment between the two groups of propolis (0.8± 0.3 mm) and Persica (2.0 ± 6.0 mm). The mean duration of ulcer healing in the Persica (5.2± 0.2) group was significantly shorter than the propolis group (0.6± 0.3) (P=0.03) (Table1).
.png)
Figure 1. Patient in Persica group before (right) and after (left) treatment.
.png)
Figure 2. Patients in Popolis group before (right) and after (left) treatment.
Table 1. The severity of pain and aphthouse of aphtous lesions before treatment, second & sixth day of treatment with Propolis & Persica mouthwashes.
| Propolis | Persica | P-value | |
| Pain severity score before treatment | 4.0 ± 3.7 | 3.0 ± 1.8 | 0.155 |
| Pain severity score in 2nd day | 5.0 ± 0.5 | 3.0 ± 4.4 | 0.287 |
| Pain severity score in 6th day | 3.0 ± 4.1 | 2.0 ± 6.0 | 0.045* |
| Size of lesions before treatment(mm) | 2.0 ± 1.3 | 3.5±0.2 | 0.107 |
| Size of lesions in 2nd day | 2.3 ±0.2 | 2.3 ±0.2 | 0.967 |
| Size of lesions in 6th day | 0.8± 0.3 | 0.2 ±0.1 | 0.104 |
The mean percentage of pain intensity reduction on the second day of treatment was not significantly different between the two groups of propolis (32.9± 0.54) and Persica (45.3±3.7) (P=0.07), but the percentage of pain intensity reduction on the sixth day of treatment in the Persica (92.4± 3.2) group was significantly higher than in the propolis group (82.8±3.2) (P=0.03) (Figure 2 & Chart 1)
The mean percentage of ulcer size reduction on the second day of treatment was not significantly different between propolis (22.9±5.5) and Persica (34.1±3.8) groups (P=0.10), but the percentage of ulcer size reduction on the sixth day of treatment in Persica (95.0±2.4) group was significantly higher than propolis group (76.0±8.9) (P=0.05), as seen in Table 2 & Chart 2. Data were reported as mean ± SEM and analyzed statistically by Man-Whitney test at a significance level (*) of P<0.05.

Chart 1. Changes in pain intensity in 2nd & 6th day of treatment in Persica & Propolis groups.
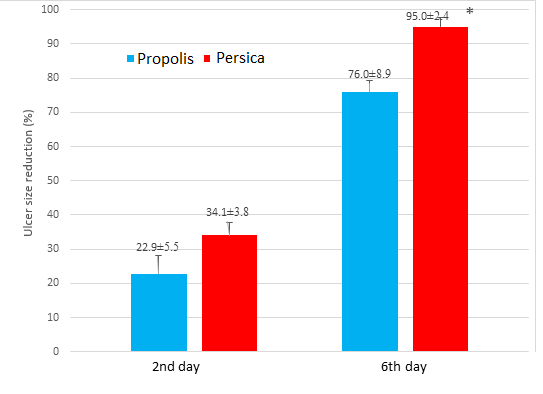

Chart 2. Changes in ulcer size in 2nd & 6th day of treatment in Persica & Propolis groups.
Table 2. Duration of healing and changes in pain intensity and size of the lesion.
| Propolis | Persica | P-value | |
| Healing duration (days) | 6.0± 0.3 | 5.2± 0.2 | *0.029 |
| Reduction of pain intensity on the 2nd day of treatment (%) | 32.9± 0.54 | 45.3±3.7 | 0.066 |
| Reduction of pain intensity on the 6th day of treatment (%) | 82.8±3.2 | 92.4± 3.2 | *0.031 |
| Ulcers size reductonn in the 2nd day of treatment (%) | 22.9±5.5 | 34.1±3.8 | 0.103 |
| Ulcers size reduction in the 6th day of treatment (%) | 76.0±8.9 | 95.0±2.4 | *0.046 |
Data were expressed as mean ± SD and analyzed by Man-Whitney statistical analysis and P <0.05 was considered significant.
Discussion
Recurrent aphthous stomatitis is a common disease of the oral cavity that affects the mucosa and soft tissues, accounting for about 15-20% of the world's population. Many drugs are currently used to treat aphthous stomatitis, most of which are chemical and have side effects, and because aphthous lesions are recurrent, repeated use of chemical compounds can cause serious complications (20, 21). Many herbal and chemical agents have been applied in the treatment of aphthous stomatitis. In the study of Rajaei-Behbahani et al. tetracycline and Myrtus communis extract has been compared as treatment of aphthous ulcers. According to this study administration of Myrtex is more effective than tetracycline in reducing the severity of pain and irritation and also improving the quality of life of patients. So, Myrtex 5% based on the mentioned method was recommended for the treatment of minor aphthous (3). The effect of propolis with minimal side effects has been investigated in the treatment of a variety of inflammatory and ulcerative diseases such as contact dermatitis (1, 22-24). In the study of Koo et al on the effects of propolis on Streptococcus mutans growth and on glucosyltransferase activity it appeared that propolis can be a promising source of new agents that may prevent dental caries and other oral diseases (22). In another study which was conducted by Shoae et al. similar results were obtained and anti-microbial effects were seen (25). Niedzielska et al. also obtained similar results and it seemed that propolis be a beneficial alternative to preparations containing chlorhexidine or triclosan (26). Momen-Beitollahi et al. showed antimicrobial effects of propolis against oral pathogens, too (27). Akhavan Karbasi et al. in another study evaluated the efficacy of propolis mouthwash in the treatment of Chemotherapy-Induced Mucositis and positive results were seen (28). In the study of Samet et al in 2007 patients were asked to take systemic propolis. It was concluded that Propolis compared to placebo diminished the recurrence rate and improved the quality of life of patients with RAS (29). Propolis has traditionally been used to treat oral ulcers. Several studies have reported the effectiveness of flavonoids in the treatment of gastrointestinal ulcers (30, 31) Casa et al evaluated the gastro protective effects of components of propolis. They concluded that propolis has positive effects in the healing of gastrointestinal ulcers (30). In the study of Boyanova et al. effects of propolis on Helicobacter pylori strains were assessed and strong and dose-dependent effects of propolis against H.pylori were seen (31). Propolis also has antimicrobial effects on oral pathogens. In the study of Sadrzadeh-Afshar et al effects of Chlorhexidine and propolis on oral pathogenes was compared. It was concluded that propolis showed acceptable results against oral microorganisms but Chlorhexidine was more effective (32). This difference can be interpreted in this way that in this study antimicrobial effects of propolis was evaluated not effects of on the aphthous ulcer which was considered in the present study.
Persica drops have been administered to prevent dental calculus, tooth decay, and bleeding gums, and as a mouthwash and periodontal pain reliever. In the study of Darbandi et al. Persica and Irsha mouth rinses were compared and had similar effects on RAS. They both had reduced pain, and duration of healing and can be prescribed for treatment of RAS. These results are similar to our study (19). Studies show that the aqueous extract of yarrow has anti-inflammatory effects due to the presence of azulene in the yarrow essential oil. Mentha has analgesic and antiseptic potential due to its menthol content. Accordingly, the present study compared the effectiveness of honey-derived mouthwash, propolis, with Persica herbal mouthwash in the treatment of minor aphthous ulcers. The duration of treatment for aphthous stomatitis is between 10-14 days.
According to the results, the healing time was 6.0 days in the propolis group and 5.2 days in the Persica group. The percentage of ulcer size reduction on the sixth day of treatment was 76% in the propolis group and 95% in the Persica group. The percentage of pain intensity reduction on the sixth day of treatment was 83% in the propolis group and 92% in the Persica group. Due to the short healing time of aphthous ulcers and pain intensity, two types of mouthwash are likely to be effective; according to results, Persica mouthwash had better effects than propolis mouthwash. Among the reasons for the better effect of Persica mouthwash in relieving pain, in the study of Abbasi et al that is in line with our study, it can be mentioned that Persica can increase salivary pH, which can justify the better analgesic effect of this mouthwash, (33). On the other hand, toothbrush trees and yarrow present in Persica contain large amounts of tannins, which can explain the analgesic and anti-irritation effects of this mouthwash due to its contractile effect on the tissue. In the study of Lewis et al. positive effects of components of persica in aspirin-induced erosions in gastric mucosa had been concluded (34). One of the etiologies of aphthous stomatitis is a weakened immune system that can occur due to an attack by bacteria and viruses. According to studies, Persica has antibacterial and antiviral properties that can strengthen the immune system and thus heal the aphthous ulcers by killing or reducing bacteria or viruses (19, 35).
According to the results, propolis mouthwash is effective in relieving pain and burning and shortening the duration of aphthous stomatitis, which based on studies and hypotheses can be attributed to the presence of caffeic acid and flavonoids in propolis through inhibiting the activity of lipoxygenase and cyclooxygenase and thus exerting an anti-inflammatory effect. It also boosts immune function by stimulating phagocytes, followed by cell-mediated immunity, which may explain the healing of aphthous stomatitis by improving one of its triggers, the weakened immune system. The various enzyme systems in propolis, such as B-complex vitamins, pro-vitamin A, arginine, and minerals such as copper, iron, zinc, and riboflavonoids, are involved in cell metabolism and collagen production, can cause ulcers to heal faster (32,36,37). Another reason for the pain relief and shortening time of ulcer healing in patients receiving propolis can be attributed to the mucosal surface covering, which prevents aphthous ulcer irritation. Slightly less effective propolis drops than Persica mouthwash can be due to the following reasons: 1- Concentration less than the effective dose of propolis mouthwash and 2- Misuse of propolis mouthwash by patients. Furthermore, some limitations were lack of cooperation of some patients and failure to visit in due time which led to the removal of those patients and admission of new ones. Also, it is recommended to determine the effect of Propolis and Persica mouthwashes on inflammatory factors involved in the pathogenesis of aphthous ulcers such as IL-6 and TNF-α in future studies.
Conclusion
Generally, both types of mouthwash had shown acceptable results with no proven side effects, the results of the present study demonstrated that the Persica mouthwash had slightly better effects in relieving pain intensity, reducing ulcer size, and shortening the duration of the disease in patients with minor aphthous ulcers compared to the Propolis mouthwash. Of course, further studies in this area are suggested for more accurate results as well as comparisons of other Therapeutic modalities.
Acknowledgments
The authors hereby express their gratitude and appreciation for the cooperation of all officials and staff of the School of Dentistry at Aja University of Medical Sciences who provided the necessary platform for this research and also for all patients participating in this study. The protocol of the study is approved by the ethics committee of Aja University of Medical Sciences. (IR.AJAUMS.REC.1398.111)
Conflict of interests
There is no conflict of interest.
Ethical Approval
The participants willingly filled out the questionnaires and signed written informed consent. The study was approved by the Ethics Committee of Aja University of Medical Sciences (code: IR.AJAUMS.REC.1398.111).
Discussion
Recurrent aphthous stomatitis is a common disease of the oral cavity that affects the mucosa and soft tissues, accounting for about 15-20% of the world's population. Many drugs are currently used to treat aphthous stomatitis, most of which are chemical and have side effects, and because aphthous lesions are recurrent, repeated use of chemical compounds can cause serious complications (20, 21). Many herbal and chemical agents have been applied in the treatment of aphthous stomatitis. In the study of Rajaei-Behbahani et al. tetracycline and Myrtus communis extract has been compared as treatment of aphthous ulcers. According to this study administration of Myrtex is more effective than tetracycline in reducing the severity of pain and irritation and also improving the quality of life of patients. So, Myrtex 5% based on the mentioned method was recommended for the treatment of minor aphthous (3). The effect of propolis with minimal side effects has been investigated in the treatment of a variety of inflammatory and ulcerative diseases such as contact dermatitis (1, 22-24). In the study of Koo et al on the effects of propolis on Streptococcus mutans growth and on glucosyltransferase activity it appeared that propolis can be a promising source of new agents that may prevent dental caries and other oral diseases (22). In another study which was conducted by Shoae et al. similar results were obtained and anti-microbial effects were seen (25). Niedzielska et al. also obtained similar results and it seemed that propolis be a beneficial alternative to preparations containing chlorhexidine or triclosan (26). Momen-Beitollahi et al. showed antimicrobial effects of propolis against oral pathogens, too (27). Akhavan Karbasi et al. in another study evaluated the efficacy of propolis mouthwash in the treatment of Chemotherapy-Induced Mucositis and positive results were seen (28). In the study of Samet et al in 2007 patients were asked to take systemic propolis. It was concluded that Propolis compared to placebo diminished the recurrence rate and improved the quality of life of patients with RAS (29). Propolis has traditionally been used to treat oral ulcers. Several studies have reported the effectiveness of flavonoids in the treatment of gastrointestinal ulcers (30, 31) Casa et al evaluated the gastro protective effects of components of propolis. They concluded that propolis has positive effects in the healing of gastrointestinal ulcers (30). In the study of Boyanova et al. effects of propolis on Helicobacter pylori strains were assessed and strong and dose-dependent effects of propolis against H.pylori were seen (31). Propolis also has antimicrobial effects on oral pathogens. In the study of Sadrzadeh-Afshar et al effects of Chlorhexidine and propolis on oral pathogenes was compared. It was concluded that propolis showed acceptable results against oral microorganisms but Chlorhexidine was more effective (32). This difference can be interpreted in this way that in this study antimicrobial effects of propolis was evaluated not effects of on the aphthous ulcer which was considered in the present study.
Persica drops have been administered to prevent dental calculus, tooth decay, and bleeding gums, and as a mouthwash and periodontal pain reliever. In the study of Darbandi et al. Persica and Irsha mouth rinses were compared and had similar effects on RAS. They both had reduced pain, and duration of healing and can be prescribed for treatment of RAS. These results are similar to our study (19). Studies show that the aqueous extract of yarrow has anti-inflammatory effects due to the presence of azulene in the yarrow essential oil. Mentha has analgesic and antiseptic potential due to its menthol content. Accordingly, the present study compared the effectiveness of honey-derived mouthwash, propolis, with Persica herbal mouthwash in the treatment of minor aphthous ulcers. The duration of treatment for aphthous stomatitis is between 10-14 days.
According to the results, the healing time was 6.0 days in the propolis group and 5.2 days in the Persica group. The percentage of ulcer size reduction on the sixth day of treatment was 76% in the propolis group and 95% in the Persica group. The percentage of pain intensity reduction on the sixth day of treatment was 83% in the propolis group and 92% in the Persica group. Due to the short healing time of aphthous ulcers and pain intensity, two types of mouthwash are likely to be effective; according to results, Persica mouthwash had better effects than propolis mouthwash. Among the reasons for the better effect of Persica mouthwash in relieving pain, in the study of Abbasi et al that is in line with our study, it can be mentioned that Persica can increase salivary pH, which can justify the better analgesic effect of this mouthwash, (33). On the other hand, toothbrush trees and yarrow present in Persica contain large amounts of tannins, which can explain the analgesic and anti-irritation effects of this mouthwash due to its contractile effect on the tissue. In the study of Lewis et al. positive effects of components of persica in aspirin-induced erosions in gastric mucosa had been concluded (34). One of the etiologies of aphthous stomatitis is a weakened immune system that can occur due to an attack by bacteria and viruses. According to studies, Persica has antibacterial and antiviral properties that can strengthen the immune system and thus heal the aphthous ulcers by killing or reducing bacteria or viruses (19, 35).
According to the results, propolis mouthwash is effective in relieving pain and burning and shortening the duration of aphthous stomatitis, which based on studies and hypotheses can be attributed to the presence of caffeic acid and flavonoids in propolis through inhibiting the activity of lipoxygenase and cyclooxygenase and thus exerting an anti-inflammatory effect. It also boosts immune function by stimulating phagocytes, followed by cell-mediated immunity, which may explain the healing of aphthous stomatitis by improving one of its triggers, the weakened immune system. The various enzyme systems in propolis, such as B-complex vitamins, pro-vitamin A, arginine, and minerals such as copper, iron, zinc, and riboflavonoids, are involved in cell metabolism and collagen production, can cause ulcers to heal faster (32,36,37). Another reason for the pain relief and shortening time of ulcer healing in patients receiving propolis can be attributed to the mucosal surface covering, which prevents aphthous ulcer irritation. Slightly less effective propolis drops than Persica mouthwash can be due to the following reasons: 1- Concentration less than the effective dose of propolis mouthwash and 2- Misuse of propolis mouthwash by patients. Furthermore, some limitations were lack of cooperation of some patients and failure to visit in due time which led to the removal of those patients and admission of new ones. Also, it is recommended to determine the effect of Propolis and Persica mouthwashes on inflammatory factors involved in the pathogenesis of aphthous ulcers such as IL-6 and TNF-α in future studies.
Conclusion
Generally, both types of mouthwash had shown acceptable results with no proven side effects, the results of the present study demonstrated that the Persica mouthwash had slightly better effects in relieving pain intensity, reducing ulcer size, and shortening the duration of the disease in patients with minor aphthous ulcers compared to the Propolis mouthwash. Of course, further studies in this area are suggested for more accurate results as well as comparisons of other Therapeutic modalities.
Acknowledgments
The authors hereby express their gratitude and appreciation for the cooperation of all officials and staff of the School of Dentistry at Aja University of Medical Sciences who provided the necessary platform for this research and also for all patients participating in this study. The protocol of the study is approved by the ethics committee of Aja University of Medical Sciences. (IR.AJAUMS.REC.1398.111)
Conflict of interests
There is no conflict of interest.
Ethical Approval
The participants willingly filled out the questionnaires and signed written informed consent. The study was approved by the Ethics Committee of Aja University of Medical Sciences (code: IR.AJAUMS.REC.1398.111).
Type of Article: Original article |
Subject:
General medicine
Received: 2021/12/5 | Accepted: 2022/02/27 | Published: 2022/03/30
Received: 2021/12/5 | Accepted: 2022/02/27 | Published: 2022/03/30
References
1. Giannetti L, Murri Dello Diago A, Lo Muzio L. Recurrent aphtous stomatitis. Minerva Stomatol. 2018 Jun;67(3):125-128. [DOI] [PMID] [Google Scholar]
2. Sereflican M, Sereflican B, Dagistan E, Goksugur N, Kizildag B. Subclinical atherosclerosis in patients with recurrent aphthous stomatitis. Oral Dis. 2016 Sep;22(6):573-7. [DOI] [PMID]
3. Rajaei-Behbahani L, Abbasi F, Sadrzadeh-Afshar M-S, Rajaei-Behbahani S, Afshar S. Effects of tetracycline and Myrtus communis extract on the treatment of recurrent aphthous ulcers: A comparative study. J Bas Res Med Sci 2021; 8(2):20-26. [Google Scholar]
4. Queiroz SIML, Silva MVAD, Medeiros AMC, Oliveira PT, Gurgel BCV, Silveira ÉJDD. Recurrent aphthous ulceration: an epidemiological study of etiological factors, treatment and differential diagnosis. An Bras Dermatol. 2018;93(3):341-346. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
5. Gholizadeh N, Sadrzadeh-Afshar MS, Sheykhbahaei N. Intralesional corticosteroid injection as an effective treatment method for oral lesions: a metaanalysis. Braz J Pharm Sci. 2020;56:e18077. [view at publisher] [DOI] [Google Scholar]
6. Wang Z, Cao H, Xiong J, Lu Y, Deng Y, Nan H, Zheng S, Ye H, Cao Z. Recent advances in the aetiology of recurrent aphthous stomatitis (RAS). Postgrad Med J. 2022 Jan;98(1155):57-66. [view at publisher] [DOI] [PMID] [Google Scholar]
7. Wang Z, Cao H, Xiong J, Lu Y, Deng Y, Nan H, Zheng S, Ye H, Cao Z. Recent advances in the aetiology of recurrent aphthous stomatitis (RAS). Postgrad Med J. 2022 Jan;98(1155):57-66. [view at publisher] [DOI] [PMID] [Google Scholar]
8. Alemrajabi MS, Dastorani M, Hamidi H, Mohammadian S, Sadrzadeh Afshar MS. Effects of Propolis and Persica Mouthwashes on Three Common Oral Streptococci: A Comparative Study. Pharm Biomed Res. 2021; 7(4):303-316. [view at publisher] [Google Scholar]
9. Kooshki F, Tabatabaei FS, Tajik S, Aayan A. The comparison of antimicrobial effects of herbal and chemical agents on toothpaste: An experimental study. Dent Res J (Isfahan). 2018 Jul-Aug;15(4):289-294. [Google Scholar]
10. Guven Y, Ustun N, Tuna EB, Aktoren O. Antimicrobial Effect of Newly Formulated Toothpastes and a Mouthrinse on Specific Microorganisms: An In Vitro Study. Eur J Dent. 2019 May;13(2):172-177. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
11. Walsh T, Worthington HV, Glenny AM, Marinho VC, Jeroncic A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst Rev. 2019;3(3):CD007868. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
12. Przybyłek I, Karpiński TM. Antibacterial Properties of Propolis. Molecules. 2019 May 29;24(11):2047. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
13. Jabbarifar SE, Tabibian SA, Poursina F. Effect of Fluoride Mouthrinse and Toothpaste on Number of Streptococcal Colony Forming Units of Dental Plaque. J Res Med Sci 2005; 10(6): 363-7. [view at publisher] [Google Scholar]
14. Sforcin JM. Biological Properties and Therapeutic Applications of Propolis. Phytother Res. 2016 Jun;30(6):894-905. [view at publisher] [DOI] [PMID] [Google Scholar]
15. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002 Nov-Dec;96(2-3):67-202. [view at publisher] [DOI] [Google Scholar]
16. S.Hariri, A., Shayesteh, S., Asgharian, P., Chamanara, M., & Sadrzadeh-Afshar, M-S. Eremostachys Binalodensis, A Potential Therapeutic Choice for Gingival Inflammatory Wounds. J Contemp Med Sci. 2021; 7(3): 140-144. [DOI]
17. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014 Dec 3;6(265):265sr6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
18. Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, Harris JD. Validation of Digital Visual Analog Scale Pain Scoring With a Traditional Paper-based Visual Analog Scale in Adults. J Am Acad Orthop Surg Glob Res Rev. 2018 Mar 23;2(3):e088. [DOI] [PMID] [PMCID] [Google Scholar]
19. Darbandi A, Nikfar F. Comparison between the two mouth rinses (Persica & antiseptic Irsha) on recurrent aphthous stomatitis. J Dent Sch. 2007; 24 (4):435-438. [Google Scholar]
20. Braakhuis A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients. 2019 Nov 8;11(11):2705. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
21. Mungmunpuntipantip R, Wiwanitkit V. Vulvar Aphthous Ulcer and COVID-19 mRNA Vaccination. J Pediatr Adolesc Gynecol. 2021 Dec 31:S1083-3188(21)00361-2. [view at publisher] [Google Scholar]
22. Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002 May; 46(5):1302-9. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
23. Khayyal MT, el-Ghazaly MA, el-Khatib AS, Hatem AM, de Vries PJ, el-Shafei S, Khattab MM. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol. 2003 Feb; 17(1):93-102. [view at publisher] [DOI] [PMID] [Google Scholar]
24. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52(4):673-751. [view at publisher] [Google Scholar]
25. Shoae Hassani AR, Hamdi K, Ghaemi A. in vitro reduction in colonization of streptococcus mutants by honey Beeswax Ethyl Acetate extract. Arak medical university Journal (Rahavard danesh ) 2009; 11 (4):87-95. [view at publisher] [Google Scholar]
26. Niedzielska I, Puszczewicz Z, Mertas A, Niedzielski D, Różanowski B, Baron S, et al. The Influence of Ethanolic Extract of Brazilian Green Propolis Gel on Hygiene and Oral Microbiota in Patients after Mandible Fractures. Biomed Res Int 2016; 2016:9190814. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
27. Momen-Beitollahi J, Mansorian A, Esmaili M, Amanlou M, Mohamadnia A, Bahrami N. Antimicrobial effects of propolis extract on the most prevalent oral pathogens: An in vitro study. J Islam Dent Assoc IRAN (JIDA). 2009; 21 (1): 33-39. [Google Scholar]
28. Akhavan Karbasi, MH, Forat Yazdi M, Ahadian H, Alili Sadrabad M. Evaluating Baremoom Mouthwash Efficacy in Treatment of Chemotherapy-Induced Mucositis. J Shahid Sadoughi Univ Med Sci 2016; 23(12): 1202-14. [view at publisher] [Google Scholar]
29. Samet N, Laurent C, Susarla SM, Samet-Rubinsteen N. The effect of bee propolis on recurrent aphthous stomatitis: a pilot study. Clin Oral Investig. 2007 Jun; 11(2):143-7. [view at publisher] [DOI] [PMID] [Google Scholar]
30. La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000 Jul; 71(1-2):45-53. [view at publisher] [DOI] [Google Scholar]
31. Boyanova L, Derejian S, Koumanova R, Katsarov N, Gergova G, Mitov I, Nikolov R, Krastev Z. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: preliminary report. J Med Microbiol. 2003 May;52(Pt 5):417-9. [view at publisher] [DOI] [PMID] [Google Scholar]
32. Sadrzadeh-Afshar M-S, Tavafi H, Niroomand S. In Vitro Effectiveness of Antimicrobial Properties of Propolis and Chlorhexidine on Oral Pathogens: A Comparative Study. Biosis: Biological Systems .2020; 1(3):116-125. [view at publisher] [DOI] [Google Scholar]
33. Abbasi F,Haghgoo R. Effect of Persica and Irsha mouth rinses on pH of saliva. Daneshvar Med. 2011; 93(7):79-84. [view at publisher] [Google Scholar]
34. Lewis DA, Shaw GP. A natural flavonoid and synthetic analogues protect the gastric mucosa from aspirin-induced erosions. J Nutr Biochem. 2001; 12(2):95-100. [view at publisher] [DOI] [Google Scholar]
35. Arshad H, Sami MA, Sadaf S, Hassan U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci Rep. 2021 Mar 16;11(1):5996. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
36. Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol 2007; 113(1): 1-14. [view at publisher] [DOI] [PMID] [Google Scholar]
37. Šuran J, Cepanec I, Mašek T, Radić B, Radić S, Tlak Gajger I, Vlainić J. Propolis Extract and Its Bioactive Compounds-From Traditional to Modern Extraction Technologies. Molecules. 2021 May 14;26(10):2930. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



