Volume 8, Issue 3 (10-2020)
Jorjani Biomed J 2020, 8(3): 44-57 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amini Najafabadi B, Keshavarz S, Asgary S, Azarbarzin M. The 8-week aerobic exercise improves blood sugar، HbA1c and lipid profile in women with type 2 diabetes: A Controlled Randomized Clinical Trial. Jorjani Biomed J 2020; 8 (3) :44-57
URL: http://goums.ac.ir/jorjanijournal/article-1-752-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-752-en.html
1- Department of Physical Education and Sports Sciences, Najafabad Branch, Islamic Azad University, Najafabad, Iran
2- Department of Physical Education and Sports Sciences, Najafabad Branch, Islamic Azad University, Najafabad, Iran/Sport Medicine Research Center, Najafabad Branch, Islamic Azad University, Najafabad, Iran ,keshavarz1357@gmail.com
3- Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
4- Nursing and Midwifery Sciences Development research Center, Najafabad Branch, Islamic Azad University, Najafabad, Iran
2- Department of Physical Education and Sports Sciences, Najafabad Branch, Islamic Azad University, Najafabad, Iran/Sport Medicine Research Center, Najafabad Branch, Islamic Azad University, Najafabad, Iran ,
3- Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
4- Nursing and Midwifery Sciences Development research Center, Najafabad Branch, Islamic Azad University, Najafabad, Iran
Keywords: Exercise [MeSH], Glycated Hemoglobin A [MeSH], lipids [MeSH], Diabetes Mellitus Type 2 [MeSH]
Full-Text [PDF 619 kb]
(3920 Downloads)
| Abstract (HTML) (8621 Views)
.png)
Discussion
The results of the present study showed a significant difference in terms of HbA1c and FBS both inter- groups (between the control and experimental groups) and within intervention group. However, there was no significant difference between the control and experimental groups in terms of mean lipid profile (LDL-C, TC, TG, BMI and HDL-C). The results of paired t-test for within group differences showed that the mean FBS, TC and HbA1c levels were significantly lower within the intervention group over time. However, the mean of these parameters increased significantly within the control group over the time. No significant intra- group differences were found for BMI, LDL-C, HDL-C and TG.
Several studies with different AE protocol and/or duration with our study also showed a significant decrease in the HbA1C level followed by PA (15, 16) which is consistent with the results of our study. HbA1C level showed significant reduction after eight weeks and sixteen weeks of AE at intensities between 50 to 85% VO2 in subjects with T2D (17, 18). In the study by Dixit. et al. the 8-week AE with different protocols from our study and different study populations, mostly male, showed similar results with our study (19) in terms of HbA1C. HbA1c is an important indicator of long-term blood sugar control (20). According to epidemiological studies HbA1c is also known as an important risk factor for CVD (21). Studies have shown that the best treatment goal in people with diabetes is to modulate HbA1c. Studies by Segalet al. (2007)، Marcus et al. (2008), and Church et al. (2010) are among the studies that confirm these results (22-24). However, these results contradict the results of Bilo et al. (2011) which they did not see any significant change in HbA1c levels after 8 weeks of AE in diabetic patients. In their study, mean age and BMI of subjects was 30-70 years and 25-40 kg/m2. These discrepancies may be due to high differences in age and BMI values (25) as well as their relatively less intensive exercises compared to the previous studies and the present study. Also, the type and method used to measure HbA1c are different in different studies (electrometry, calorimetry, chromatography), which can affect the results (26). AE can increase glucose transporters, which improves insulin function and glucose metabolism, and can lower HbA1c.
PA also ensures homeostasis of glucose and glycemic factors by decreasing FBS levels. The results of the present study showed a significant decrease in FBS values both inter- group and also intra-group over time which is consistent with the results of Misra et al (2008), Cauza et al (2005), Shenoy et al (2009) (27-29). Tekmadikis et al. during a different AE protocol (4-16 weeks AE) found a significant decrease in FBS levels and an improvement in insulin sensitivity in type 2 diabetic subjects (30). On the other hand, Cauza et al. did not show any significant reduction in blood sugar after 4 months of AE. It might be due to the fact that the duration of AE in each session (15-30 minutes) was relatively short in their study (28). In a study by Rahbar et al. with very similar AE duration time (8 weeks) and AE protocol (3 sessions per week, intensities between 50%- 70%) with our study significant changes in HbA1c and FBS inter-group were observed however contrary to our study they also found significant changes in cholesterol, LDL-C and TG compared to control. They interestingly reported the elevation of HDL-C in control group compared to intervention (31). Considering the similar AE protocole between their study with our the difference in the effect of intervention on lipid profile needs more research.
One possible mechanism that explains the role of AE in controlling and lowering blood sugar (FBS and HbA1c) in type 2 diabetes is an increase in Glut4 counts, which will be resulted in increased glucose uptake into muscle cells and glucose uptake. Muscles expend large amounts of glucose at two times, one during insulin-dependent PA and another one about 2-3 hours after each meal. Frequent muscle contractions during exercise have an insulin-like effect and send large amounts of glucose into the cell to produce energy. These frequent contractions increase the number of Glut4 molecules and increase transportation of glucose across the cell membrane. It also allows muscle fibers to have low glycogen concentrations for a relatively long period of time (32). On the other hand, muscle cells try to regenerate their glycogen reserves after exercise, and for this reason, the blood glucose concentration is at a low level for several hours after exercise. Research shows that Glut4 levels in young athletes are higher than non-athletes people. Approximately, 80% of patients with type 2 diabetes are obese and obesity is a major cause of insulin resistance (33). On the other hand, fatty acids produced by adipose tissue disrupt the transfer of Glut4 to the cell surface (34). Exercise may prevent fatty acids from accumulating in muscle cells by increasing oxidation. Increased capillary density, increased sensitivity of insulin receptors, changes in phospholipid composition of sarcolemma, increased activity of oxidative enzymes, and increased activity of glycogen synthase are also among the important factors in lowering blood sugar (29). A study showed that 8-week AE improved insulin resistance within the experimental group and also between the groups significantly (35).
Optimal LDL-C levels for adults with diabetes are <100 mg/dl (2.60 mmol/l); HDL-C are >40 mg/dl (1.02 mmol/l); and TG levels are <150 mg/dl (1.7 mmol/l). In women, who tend to have higher HDL cholesterol levels than men, an HDL goal 10 mg/dl higher may be appropriate (36). The results of the present study showed no significant difference between the groups in terms of lipid profile following AE. However, the results of paired t-test for intra group analysis showed that TC level decreased significantly within the experimental group over time. There was no significant difference inter-group or intra group within experimental group in terms of TG, LDL-C, and HDL-C in our study which might be due to the insufficient intensity and duration of AE in our protocol, lack of dietary fat restriction and diet, or could be due to the genetically factors, along with environmental influences. A review of 51 articles on the effect of PA intervention on non-diabetic subjects showed a mean increase in HDL cholesterol of 4.6 % however different study and meta-analysis on the effect of PA on diabetic subjects did not confirm this improvement in HDL-C (12, 35).
Although our study population was only female diabetic patients and a study showed that the level of performing PA is lower in women than in men (37) the results of the present study were consistent with a number of various reports that have not seen positive effect of applying AE diabetic patients on TG, LDL-C, and HDL-C levels (22, 25, 28, 38). There is strong evidence that high-intensity exercise exerts significant positive effects on lipid profile and decreases mortality rate by more than double over a decade (39). The intensity, duration, and frequency of exercises can be considered as the reason for achieving these results. Also, the reduction of saturated fats, diet, and weight loss has been considered to affect lipid changes (40, 41). Contrary to the results of the present study, Baharloo et al. (2014) stated that a 12-week aerobic exercise led to a significant decrease in TG, LDL, and HDL levels. Researchers believe that the lipid profile levels at the beginning of exercise are influential, so that the higher the level of blood lipids, the more noticeable changes will be shown (17, 42, 43). The absence of any change in the lipid profile in the present study may be attributed to the low initial levels of this variable.
The mostly used measure of cholesterol is ‘total cholesterol’ or TC, which includes LDL-C and HDL-C (44). We found a significant decrease in TC level following aerobic activity. Elevated TC level increases risk of CVD related problems approximately twice in non-diabetic patients (45) and the mean annual CVD related mortality as three times in patients with elevated cholesterol levels (>220 mg/dL)(46). therefore, these results confirm the cardio-protective effect of PA for this group of patients (47).
The mechanism by which exercise activity improves fat metabolism may be due to changes in the activity of lipase enzymes, including lipoprotein lipase (LPL) and hormone-sensitive lipase (HSL). One of the possible reasons for the decrease in TC in the present study is increased LPL activity (48). Researchers have found that long-term PA has a greater effect on lowering blood cholesterol levels than intense and short-term exercises. Every diabetic patient should engage in at least 150 min moderate intensity PA per week, at least 3 days/week, with no more than two consecutive days without activity (49).
Conclusion
The results of present study support the undeniable beneficial effects of physical activity in patients with type 2 diabetes. In this study, aerobic exercises played a significant role in reducing HbA1c and FBS levels in inter-group and intra-group analysis. Aerobic exercise also led to a decrease in TC level in intervention group over time. However, the results showed no beneficial effect for 8-week aerobic exercises in term of TG, LDL-C and HDL-C. Based on the results of this study it can be concluded that aerobic exercises has blood glucose management and cardiovascular protective activity.
Due to the existing limitations such as: budget, it was not possible to measure insulin and insulin resistance in the subjects. Therefore, it is suggested that serum insulin and insulin resistance be evaluated in diabetics simultaneously with the evaluation of glycemic index.
Acknowledgments
This article is part of a student dissertation. Hereby, the author would like to express their gratitude to the staff working in Janan Diabetes Center and the Nabi Akram Laboratory in Najafabad as well as all the diabetic women participating in this research project.
Declarations
A written informed consent was obtained from all participants. The study was approved by randomized clinical trial at the Iranian Clinical Trial Registration Center. The registration code number is IRCT20200803048287N1 and ethics code was: IR.IAU.NAJAFABAD.REC.1399/083.
Full-Text: (3064 Views)
Introduction
Diabetes is a chronic metabolic disorder associated with insufficient insulin production and elevated blood sugar levels (hyperglycemia) as a result. Type 2 diabetes (T2D) is associated with many cardiovascular diseases (CVD) related risk factors such as obesity, hypertension, hyperlipidemia, lack of physical activity, elevated FBS, smoking and elevated glycosylated hemoglobin (hemoglobin A1c) (1-3). T2D causes 2- 4 fold higher risk factor for CVD related death (4). Regular AE is considered as a treatment program for CVD and reduces CVD related complications (5). Regular physical activity (PA) also improves glucose and lipid metabolism by increasing insulin sensitivity. PA increases HDL-C and reduces TG and LDL-C levels. PA can increase the body's response to insulin and increases insulin sensitivity and found to be useful in preventing T2D, FBS management (6). Recent studies found that AE improves blood sugar by reducing HbA1c levels, increases insulin sensitivity, and plays an important role in controlling diabetes and preventing subsequent cardiac complications (7-10).
Aerobic exercise (AE) increases insulin receptor uptake and glucose transportion into muscle tissue (7). The American Diabetes Association (ADA) recommends 30-min moderate aerobic activity for 5 days of a week or 150 minutes per week (11). Previous studies show that AE is effective in term of controlling diabetes by activating AMPK pathway and increasing glucose uptake. AMPK activity increases glucose transport by increasing the serum GLUT4 level in insulin-resistant skeletal muscle and mediates the effects of GLUT4 expression.
So far many investigations have been performed on the effect of AE on T2D subjects and one meta-analysis reviewed 21 articles on this topic (12). According to this met-analysis the most common reason for exclusion of studies was lack of control group. Most of the studies prescribing AE three days per week, 50 and 85% Vo2 peak, 55 and 85% maximum heart rate, and the length of AE intervention ranged between 8 weeks to 1 year. Results on the effect of AE on diabetics are still heterogeneous, and more controlled randomized trials with different AE protocols on different populations are needed to reach more conclusive conclusions with less heterogeneity. Therefore the present controlled randomized clinical trial designed to investigate the effect of 8-week AE on FBS,A1c (HbA1c) and lipid profile [triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C)]in T2D female patients. The female population was actually selected because they were more accessible. To the best of our knowledge this study is unique in term of AE protocol (HR max (55-75%, three sessions AE per week for 8 weeks, each session 50 minutes with moderate-intensity AE) and study population (only female diabetic subjects were considered).
Materials and Methods
This was a randomized controlled clinical trial carried out on selected 30 women with type 2 diabetes (age = 30-50 years). Inclusion criteria included the presence of T2D based on the criterion of the world health organization (WHO). FBS (≥7 mmol / L, (126 mg / dL) and 1HbA1c values (≥11mmol/mol), having diabetes for more than three years, undergoing treatment only with oral drugs and no insulin administration, absence of regular PA for at least the last 6 months and not having any major disease (including history of CVD, history of heart surgery) affecting research variables and non-use of tobacco, alcohol, caffeine, and drugs. First, the necessary information about the nature and methodology of the research were provided to the subjects. Participants then completed and signed the consent form to participate in the research. After completing the medical history questionnaire, people with heart disease and hypertension were excluded. The sample size was calculated by considering the reliability coefficient of 0.95, statistical power of 0.80 according to the following formula:.png)
Diabetes is a chronic metabolic disorder associated with insufficient insulin production and elevated blood sugar levels (hyperglycemia) as a result. Type 2 diabetes (T2D) is associated with many cardiovascular diseases (CVD) related risk factors such as obesity, hypertension, hyperlipidemia, lack of physical activity, elevated FBS, smoking and elevated glycosylated hemoglobin (hemoglobin A1c) (1-3). T2D causes 2- 4 fold higher risk factor for CVD related death (4). Regular AE is considered as a treatment program for CVD and reduces CVD related complications (5). Regular physical activity (PA) also improves glucose and lipid metabolism by increasing insulin sensitivity. PA increases HDL-C and reduces TG and LDL-C levels. PA can increase the body's response to insulin and increases insulin sensitivity and found to be useful in preventing T2D, FBS management (6). Recent studies found that AE improves blood sugar by reducing HbA1c levels, increases insulin sensitivity, and plays an important role in controlling diabetes and preventing subsequent cardiac complications (7-10).
Aerobic exercise (AE) increases insulin receptor uptake and glucose transportion into muscle tissue (7). The American Diabetes Association (ADA) recommends 30-min moderate aerobic activity for 5 days of a week or 150 minutes per week (11). Previous studies show that AE is effective in term of controlling diabetes by activating AMPK pathway and increasing glucose uptake. AMPK activity increases glucose transport by increasing the serum GLUT4 level in insulin-resistant skeletal muscle and mediates the effects of GLUT4 expression.
So far many investigations have been performed on the effect of AE on T2D subjects and one meta-analysis reviewed 21 articles on this topic (12). According to this met-analysis the most common reason for exclusion of studies was lack of control group. Most of the studies prescribing AE three days per week, 50 and 85% Vo2 peak, 55 and 85% maximum heart rate, and the length of AE intervention ranged between 8 weeks to 1 year. Results on the effect of AE on diabetics are still heterogeneous, and more controlled randomized trials with different AE protocols on different populations are needed to reach more conclusive conclusions with less heterogeneity. Therefore the present controlled randomized clinical trial designed to investigate the effect of 8-week AE on FBS,A1c (HbA1c) and lipid profile [triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C)]in T2D female patients. The female population was actually selected because they were more accessible. To the best of our knowledge this study is unique in term of AE protocol (HR max (55-75%, three sessions AE per week for 8 weeks, each session 50 minutes with moderate-intensity AE) and study population (only female diabetic subjects were considered).
Materials and Methods
This was a randomized controlled clinical trial carried out on selected 30 women with type 2 diabetes (age = 30-50 years). Inclusion criteria included the presence of T2D based on the criterion of the world health organization (WHO). FBS (≥7 mmol / L, (126 mg / dL) and 1HbA1c values (≥11mmol/mol), having diabetes for more than three years, undergoing treatment only with oral drugs and no insulin administration, absence of regular PA for at least the last 6 months and not having any major disease (including history of CVD, history of heart surgery) affecting research variables and non-use of tobacco, alcohol, caffeine, and drugs. First, the necessary information about the nature and methodology of the research were provided to the subjects. Participants then completed and signed the consent form to participate in the research. After completing the medical history questionnaire, people with heart disease and hypertension were excluded. The sample size was calculated by considering the reliability coefficient of 0.95, statistical power of 0.80 according to the following formula:
.png)
A random block was used to determine the sample for each group and the blind method was one-way.
Experimental design
Prior to the study, the groups were compared matched for age, height, and weight and no significant difference were found between them. Subjects were then randomly divided into two groups: experimental group consisted of 15 diabetic women participating in moderate-intensity aerobic exercise and control group consisted of 15 diabetic women who did not engage in regular exercise.
Interventions
The control group did not do any AE. The exercise program in the intervention group included aerobic exercises, 3 sessions per week for 8 weeks. Moderate-intensity AE (walking), was performed at HR max (55-75%). HR max was estimated using Karvonen formula (Maximum Heart Rate: 220- your age) and heart rate of the subjects during exercise were controlled by taking the pulse from the carotid area. These exercises were performed with perceived exertion ratings between12 to 13 for 150 minutes per week and 50 minutes per session. The warm-up program included 10 minutes of jogging, combined arm and leg movements, and stretching exercises. Aerobic exercises were performed for 35 minutes in the first two weeks and 5 minutes were added to the training time once every two weeks by observing the principle of progressive overload. Also, to return the body to its initial state, cooling movements were performed for 10 minutes. Exercises were performed in a gym at a temperature of 25 °C. The exercise protocol was implemented according to the research conducted by Horden et al. (2012) and Mendes et al. (2016) (Table 1)(13, 14).
Table 1: Exercise protocol
Experimental design
Prior to the study, the groups were compared matched for age, height, and weight and no significant difference were found between them. Subjects were then randomly divided into two groups: experimental group consisted of 15 diabetic women participating in moderate-intensity aerobic exercise and control group consisted of 15 diabetic women who did not engage in regular exercise.
Interventions
The control group did not do any AE. The exercise program in the intervention group included aerobic exercises, 3 sessions per week for 8 weeks. Moderate-intensity AE (walking), was performed at HR max (55-75%). HR max was estimated using Karvonen formula (Maximum Heart Rate: 220- your age) and heart rate of the subjects during exercise were controlled by taking the pulse from the carotid area. These exercises were performed with perceived exertion ratings between12 to 13 for 150 minutes per week and 50 minutes per session. The warm-up program included 10 minutes of jogging, combined arm and leg movements, and stretching exercises. Aerobic exercises were performed for 35 minutes in the first two weeks and 5 minutes were added to the training time once every two weeks by observing the principle of progressive overload. Also, to return the body to its initial state, cooling movements were performed for 10 minutes. Exercises were performed in a gym at a temperature of 25 °C. The exercise protocol was implemented according to the research conducted by Horden et al. (2012) and Mendes et al. (2016) (Table 1)(13, 14).
Table 1: Exercise protocol
| Intensity | Period | week |
| 55-60% | 35 | The first and second week |
| 60-65 % | 40 | Third and fourth week |
| 65-70 % | 45 | Fifth and sixth week |
| 70-75 % | 50 | Seventh and eighth week |
Anthropometrics
In this study, the height of people was measured by means of a SECA (US) portable stadiometer with a 0.1cm precision and weight variable was also measured based on kilograms by Beurer model scales while people had the least clothes and no shoes.
Blood analysis
The subjects were introduced to Nabi Akram Laboratory in Najafabad where they were asked to rest in a sitting position for 20 minutes. Blood samples were taken from the brachial vein (5ml) to measure glycemic index and lipid profile after 12 hours of overnight fasting in the pre-test and in the post-test (48 hours after the last exercise session). The centrifuged blood samples and collected serum were then stored for further analysis. Lipid profile levels were measured by an autoanalyzer (BA400) using a Biosystem kit and peroxidase method. FBS level was measured by a biosystem kit and an autoanalyzer (BA400) using glucose oxidase method. HbA1C level was also measured by Axzshzld kit and autoanalyzer (BA400) using immunoturbidimetry method.
Statistical analysis
Descriptive data were reported as mean ± standard deviation. Intergroup and intragroup changes were compared using Independent t-test and paired t-test, respectively. P ≤ 0.05 was considered as the significance level and data analysis was performed using SPSS ver. 22.
Result
Table 2 shows statistical data related to the demographic characteristics of the subjects and the mean of glycemic indexes and lipid profile at the baseline (pre-test) in the research groups. The results of independent t-test showed no significant difference between the two groups in terms of height, weight and age (p> 0.05) and the two groups are homogeneous in all of the above cases. The results of this test also showed no difference between the two groups in terms of the mean , BMI , HbA1c, FBS, TG, TC, LDL-C, HDL-C and levels at the beginning of the study and the two groups were homogeneous in terms of these indices (p>0.05).
In this study, the height of people was measured by means of a SECA (US) portable stadiometer with a 0.1cm precision and weight variable was also measured based on kilograms by Beurer model scales while people had the least clothes and no shoes.
Blood analysis
The subjects were introduced to Nabi Akram Laboratory in Najafabad where they were asked to rest in a sitting position for 20 minutes. Blood samples were taken from the brachial vein (5ml) to measure glycemic index and lipid profile after 12 hours of overnight fasting in the pre-test and in the post-test (48 hours after the last exercise session). The centrifuged blood samples and collected serum were then stored for further analysis. Lipid profile levels were measured by an autoanalyzer (BA400) using a Biosystem kit and peroxidase method. FBS level was measured by a biosystem kit and an autoanalyzer (BA400) using glucose oxidase method. HbA1C level was also measured by Axzshzld kit and autoanalyzer (BA400) using immunoturbidimetry method.
Statistical analysis
Descriptive data were reported as mean ± standard deviation. Intergroup and intragroup changes were compared using Independent t-test and paired t-test, respectively. P ≤ 0.05 was considered as the significance level and data analysis was performed using SPSS ver. 22.
Result
Table 2 shows statistical data related to the demographic characteristics of the subjects and the mean of glycemic indexes and lipid profile at the baseline (pre-test) in the research groups. The results of independent t-test showed no significant difference between the two groups in terms of height, weight and age (p> 0.05) and the two groups are homogeneous in all of the above cases. The results of this test also showed no difference between the two groups in terms of the mean , BMI , HbA1c, FBS, TG, TC, LDL-C, HDL-C and levels at the beginning of the study and the two groups were homogeneous in terms of these indices (p>0.05).
Table 2. Demographic information of research subjects
| P | T | M± SD | group | Variable |
| 0.86 | 0.186 | 7±51 | Control Experimental |
Age (years) |
| 3±50 | ||||
| 0.81 | 0.230 | 0.04±1.61 | Control Experimental |
Height (cm) |
| 0.05±1.59 | ||||
| 0.14 0.489 |
1.505 | 6.2±70.1 | Control Experimental Control Experimental |
Body Weight (kg) BMI (pree-xam) |
| 0.701- |
6.2±69.5 2.33± 27.10 2± 27.65 |
|||
| 0.57 | -0.575 | 59.03±174.93 | Control Experimental |
FBS (pree-xam) |
| 51.85±186.60 | ||||
| 0.83 | -0.213 | 1.53± 7.68 | Control Experimental |
HbA1c (pree-xam) |
| 1.88±7.82 | ||||
| 0.71 | 0.375 | 61.05±152.53 | Control Experimental |
Triglyceride (pree-xam) |
| 65.07±128.13 | ||||
| 0.47 | 0.723 | 61.05±152.53 | Control Experimental |
Cholesterol (pree-xam) |
| 34.33±163.87 | ||||
| 0.33 | 0.979 | 21.71±101.60 | Control Experimental |
L.D.L (pree-xam) |
| 31.10±88.40 | ||||
| 0.26 | - | 6.86±49 | Control Experimental |
*HDL (pree-xam) |
| 4.57±47.47 | ||||
| Mann-Whitney test was used to evaluate this index* | ||||
First, paired t-test was used to compare intragroup changes in lipid profile (LDL-C, TG, TC), BMI, FBS and HbA1c in the pre-test and post-test phases. Wilcoxon test was also used for HDL index.
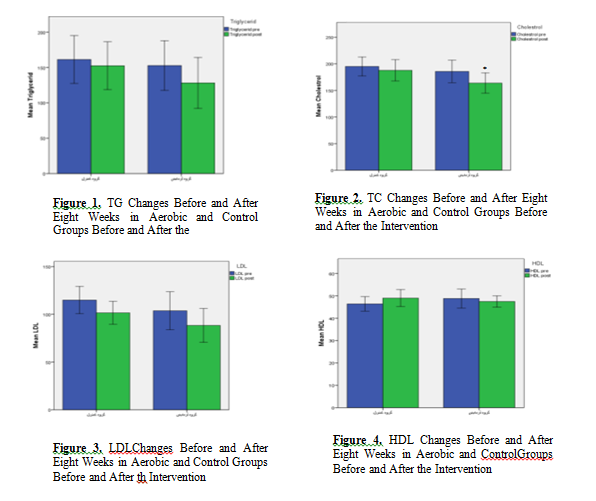
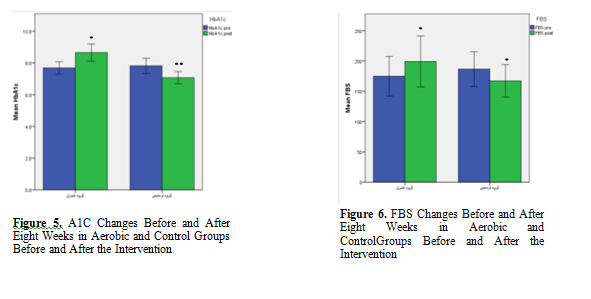
The results of paired t-test showed that the mean FBS and HbA1c levels in experimental group were significantly lower in the post-test phase (p = 0.03) as compared to the pre-test phase (p = 0.002), however, the mean of these parameters showed a significant increase in the control group in post-test (p = 0.03) as compared to the pre-test phase (p = 0.05). Cholesterol (TC) level did not change significantly within the control group but its amount within the experimental group decreased significantly after AE (p= 0.05). There was no significant difference between the control and experimental groups in terms of BMI,TG, LDL-C, and HDL-C levels before and after the intervention (p> 0.05).
Independent t-test was also used to compare intergroup changes in terms of LDL-C, TG, TC, HbA1c, FBS ,BMI and Mann-Whitney was also used to compare changes in HDL-C level between the two groups. The results of these tests showed significant difference between the two groups in terms of mean values of HbA1c and FBS levels in the post-test (p <0.05). Figure 1-7.
The results of paired t-test showed that the mean FBS and HbA1c levels in experimental group were significantly lower in the post-test phase (p = 0.03) as compared to the pre-test phase (p = 0.002), however, the mean of these parameters showed a significant increase in the control group in post-test (p = 0.03) as compared to the pre-test phase (p = 0.05). Cholesterol (TC) level did not change significantly within the control group but its amount within the experimental group decreased significantly after AE (p= 0.05). There was no significant difference between the control and experimental groups in terms of BMI,TG, LDL-C, and HDL-C levels before and after the intervention (p> 0.05).
Independent t-test was also used to compare intergroup changes in terms of LDL-C, TG, TC, HbA1c, FBS ,BMI and Mann-Whitney was also used to compare changes in HDL-C level between the two groups. The results of these tests showed significant difference between the two groups in terms of mean values of HbA1c and FBS levels in the post-test (p <0.05). Figure 1-7.


.png)
Discussion
The results of the present study showed a significant difference in terms of HbA1c and FBS both inter- groups (between the control and experimental groups) and within intervention group. However, there was no significant difference between the control and experimental groups in terms of mean lipid profile (LDL-C, TC, TG, BMI and HDL-C). The results of paired t-test for within group differences showed that the mean FBS, TC and HbA1c levels were significantly lower within the intervention group over time. However, the mean of these parameters increased significantly within the control group over the time. No significant intra- group differences were found for BMI, LDL-C, HDL-C and TG.
Several studies with different AE protocol and/or duration with our study also showed a significant decrease in the HbA1C level followed by PA (15, 16) which is consistent with the results of our study. HbA1C level showed significant reduction after eight weeks and sixteen weeks of AE at intensities between 50 to 85% VO2 in subjects with T2D (17, 18). In the study by Dixit. et al. the 8-week AE with different protocols from our study and different study populations, mostly male, showed similar results with our study (19) in terms of HbA1C. HbA1c is an important indicator of long-term blood sugar control (20). According to epidemiological studies HbA1c is also known as an important risk factor for CVD (21). Studies have shown that the best treatment goal in people with diabetes is to modulate HbA1c. Studies by Segalet al. (2007)، Marcus et al. (2008), and Church et al. (2010) are among the studies that confirm these results (22-24). However, these results contradict the results of Bilo et al. (2011) which they did not see any significant change in HbA1c levels after 8 weeks of AE in diabetic patients. In their study, mean age and BMI of subjects was 30-70 years and 25-40 kg/m2. These discrepancies may be due to high differences in age and BMI values (25) as well as their relatively less intensive exercises compared to the previous studies and the present study. Also, the type and method used to measure HbA1c are different in different studies (electrometry, calorimetry, chromatography), which can affect the results (26). AE can increase glucose transporters, which improves insulin function and glucose metabolism, and can lower HbA1c.
PA also ensures homeostasis of glucose and glycemic factors by decreasing FBS levels. The results of the present study showed a significant decrease in FBS values both inter- group and also intra-group over time which is consistent with the results of Misra et al (2008), Cauza et al (2005), Shenoy et al (2009) (27-29). Tekmadikis et al. during a different AE protocol (4-16 weeks AE) found a significant decrease in FBS levels and an improvement in insulin sensitivity in type 2 diabetic subjects (30). On the other hand, Cauza et al. did not show any significant reduction in blood sugar after 4 months of AE. It might be due to the fact that the duration of AE in each session (15-30 minutes) was relatively short in their study (28). In a study by Rahbar et al. with very similar AE duration time (8 weeks) and AE protocol (3 sessions per week, intensities between 50%- 70%) with our study significant changes in HbA1c and FBS inter-group were observed however contrary to our study they also found significant changes in cholesterol, LDL-C and TG compared to control. They interestingly reported the elevation of HDL-C in control group compared to intervention (31). Considering the similar AE protocole between their study with our the difference in the effect of intervention on lipid profile needs more research.
One possible mechanism that explains the role of AE in controlling and lowering blood sugar (FBS and HbA1c) in type 2 diabetes is an increase in Glut4 counts, which will be resulted in increased glucose uptake into muscle cells and glucose uptake. Muscles expend large amounts of glucose at two times, one during insulin-dependent PA and another one about 2-3 hours after each meal. Frequent muscle contractions during exercise have an insulin-like effect and send large amounts of glucose into the cell to produce energy. These frequent contractions increase the number of Glut4 molecules and increase transportation of glucose across the cell membrane. It also allows muscle fibers to have low glycogen concentrations for a relatively long period of time (32). On the other hand, muscle cells try to regenerate their glycogen reserves after exercise, and for this reason, the blood glucose concentration is at a low level for several hours after exercise. Research shows that Glut4 levels in young athletes are higher than non-athletes people. Approximately, 80% of patients with type 2 diabetes are obese and obesity is a major cause of insulin resistance (33). On the other hand, fatty acids produced by adipose tissue disrupt the transfer of Glut4 to the cell surface (34). Exercise may prevent fatty acids from accumulating in muscle cells by increasing oxidation. Increased capillary density, increased sensitivity of insulin receptors, changes in phospholipid composition of sarcolemma, increased activity of oxidative enzymes, and increased activity of glycogen synthase are also among the important factors in lowering blood sugar (29). A study showed that 8-week AE improved insulin resistance within the experimental group and also between the groups significantly (35).
Optimal LDL-C levels for adults with diabetes are <100 mg/dl (2.60 mmol/l); HDL-C are >40 mg/dl (1.02 mmol/l); and TG levels are <150 mg/dl (1.7 mmol/l). In women, who tend to have higher HDL cholesterol levels than men, an HDL goal 10 mg/dl higher may be appropriate (36). The results of the present study showed no significant difference between the groups in terms of lipid profile following AE. However, the results of paired t-test for intra group analysis showed that TC level decreased significantly within the experimental group over time. There was no significant difference inter-group or intra group within experimental group in terms of TG, LDL-C, and HDL-C in our study which might be due to the insufficient intensity and duration of AE in our protocol, lack of dietary fat restriction and diet, or could be due to the genetically factors, along with environmental influences. A review of 51 articles on the effect of PA intervention on non-diabetic subjects showed a mean increase in HDL cholesterol of 4.6 % however different study and meta-analysis on the effect of PA on diabetic subjects did not confirm this improvement in HDL-C (12, 35).
Although our study population was only female diabetic patients and a study showed that the level of performing PA is lower in women than in men (37) the results of the present study were consistent with a number of various reports that have not seen positive effect of applying AE diabetic patients on TG, LDL-C, and HDL-C levels (22, 25, 28, 38). There is strong evidence that high-intensity exercise exerts significant positive effects on lipid profile and decreases mortality rate by more than double over a decade (39). The intensity, duration, and frequency of exercises can be considered as the reason for achieving these results. Also, the reduction of saturated fats, diet, and weight loss has been considered to affect lipid changes (40, 41). Contrary to the results of the present study, Baharloo et al. (2014) stated that a 12-week aerobic exercise led to a significant decrease in TG, LDL, and HDL levels. Researchers believe that the lipid profile levels at the beginning of exercise are influential, so that the higher the level of blood lipids, the more noticeable changes will be shown (17, 42, 43). The absence of any change in the lipid profile in the present study may be attributed to the low initial levels of this variable.
The mostly used measure of cholesterol is ‘total cholesterol’ or TC, which includes LDL-C and HDL-C (44). We found a significant decrease in TC level following aerobic activity. Elevated TC level increases risk of CVD related problems approximately twice in non-diabetic patients (45) and the mean annual CVD related mortality as three times in patients with elevated cholesterol levels (>220 mg/dL)(46). therefore, these results confirm the cardio-protective effect of PA for this group of patients (47).
The mechanism by which exercise activity improves fat metabolism may be due to changes in the activity of lipase enzymes, including lipoprotein lipase (LPL) and hormone-sensitive lipase (HSL). One of the possible reasons for the decrease in TC in the present study is increased LPL activity (48). Researchers have found that long-term PA has a greater effect on lowering blood cholesterol levels than intense and short-term exercises. Every diabetic patient should engage in at least 150 min moderate intensity PA per week, at least 3 days/week, with no more than two consecutive days without activity (49).
Conclusion
The results of present study support the undeniable beneficial effects of physical activity in patients with type 2 diabetes. In this study, aerobic exercises played a significant role in reducing HbA1c and FBS levels in inter-group and intra-group analysis. Aerobic exercise also led to a decrease in TC level in intervention group over time. However, the results showed no beneficial effect for 8-week aerobic exercises in term of TG, LDL-C and HDL-C. Based on the results of this study it can be concluded that aerobic exercises has blood glucose management and cardiovascular protective activity.
Due to the existing limitations such as: budget, it was not possible to measure insulin and insulin resistance in the subjects. Therefore, it is suggested that serum insulin and insulin resistance be evaluated in diabetics simultaneously with the evaluation of glycemic index.
Acknowledgments
This article is part of a student dissertation. Hereby, the author would like to express their gratitude to the staff working in Janan Diabetes Center and the Nabi Akram Laboratory in Najafabad as well as all the diabetic women participating in this research project.
Declarations
A written informed consent was obtained from all participants. The study was approved by randomized clinical trial at the Iranian Clinical Trial Registration Center. The registration code number is IRCT20200803048287N1 and ethics code was: IR.IAU.NAJAFABAD.REC.1399/083.
Type of Article: Original article |
Subject:
Health
Received: 2020/07/18 | Accepted: 2020/08/6 | Published: 2020/10/1
Received: 2020/07/18 | Accepted: 2020/08/6 | Published: 2020/10/1
References
1. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical diabetes. 2008;26(2):77-82. [view at publisher] [DOI] [Google Scholar]
2. Gerich JE. Type 2 diabetes mellitus is associated with multiple cardiometabolic risk factors. Clinical cornerstone. 2007;8(3):53-68. [view at publisher] [DOI] [Google Scholar]
3. Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976-86. [view at publisher] [DOI] [Google Scholar]
4. AD A. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8-S16. [view at publisher] [DOI] [Google Scholar]
5. Napoli C, Balestrieri A, Ignarro LJ. Therapeutic approaches in vascular repair induced by adult bone marrow cells and circulating progenitor endothelial cells. Current pharmaceutical design. 2007;13(31):3245-51. [view at publisher] [DOI] [Google Scholar]
6. Medicine ACoS. American Diabetes Association (ADA). Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42(12):2282-303. [view at publisher] [DOI] [Google Scholar]
7. Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes care. 2006;29(8):1933-41. [view at publisher] [DOI] [Google Scholar]
8. Stewart K. Exercise training: can it improve cardiovascular health in patients with type 2 diabetes? British journal of sports medicine. 2004;38(3):250-2. [view at publisher] [DOI] [Google Scholar]
9. Alam S, Stolinski M, Pentecost C, Boroujerdi MA, Jones RH, Sonksen PH, et al. The effect of a six-month exercise program on very low-density lipoprotein apolipoprotein B secretion in type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2004;89(2):688-94. [view at publisher] [DOI] [Google Scholar]
10. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes care. 2004;27(10):2518-39. [view at publisher] [DOI] [Google Scholar]
11. Rossi G. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2018;33(Suppl 1):S62-S9. [view at publisher] [DOI] [Google Scholar]
12. Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes care. 2011;34(5):1228-37. [view at publisher] [DOI] [Google Scholar]
13. Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MAF, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. Journal of Science and Medicine in Sport. 2012;15(1):25-31. [view at publisher] [DOI] [Google Scholar]
14. Mendes R, Sousa N, Almeida A, Subtil P, Guedes-Marques F, Reis VM, et al. Exercise prescription for patients with type 2 diabetes-a synthesis of international recommendations: narrative review. British journal of sports medicine. 2016;50(22):1379-81. [view at publisher] [DOI] [Google Scholar]
15. Yavari A, Hajiyev A, Naghizadeh F. The effect of aerobic exercise on glycosylated hemoglobin values in type 2 diabetes patients. Journal of sports medicine and physical fitness. 2010;50(4):501. [Google Scholar]
16. Najafipour F, Mobasseri M, Yavari A, Nadrian H, Aliasgarzadeh A, Abbasi NM, et al. Effect of regular exercise training on changes in HbA1c, BMI and VO2max among patients with type 2 diabetes mellitus: an 8-year trial. BMJ Open Diabetes Research and Care. 2017;5(1). [view at publisher] [DOI] [Google Scholar]
17. Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes care. 2007;30(3):719-21. [view at publisher] [DOI] [Google Scholar]
18. Yousefipoor P, Tadibi V, Behpoor N, Parnow A, Delbari M, Rashidi S. Effects of aerobic exercise on glucose control and cardiovascular risk factor in type 2 diabetes patients. medical journal of mashhad university of medical sciences. 2015;57(9):976-84. [view at publisher] [Google Scholar]
19. Dixit S, Maiya A, Shastry B. Effect of moderate-intensity aerobic exercise on glycosylated haemoglobin among elderly patients with type 2 diabetes & peripheral neuropathy. The Indian journal of medical research. 2017;145(1):129. [view at publisher] [DOI] [Google Scholar]
20. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomarker insights. 2016;11:BMI. S38440. [view at publisher] [DOI] [Google Scholar]
21. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New England Journal of Medicine. 2010;362(9):800-11. [view at publisher] [DOI] [Google Scholar]
22. Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Annals of internal medicine. 2007;147(6):357-69. [view at publisher] [DOI] [Google Scholar]
23. Marcus RL, Smith S, Morrell G, Addison O, Dibble LE, Wahoff-Stice D, et al. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Physical therapy. 2008;88(11):1345-54. [view at publisher] [DOI] [Google Scholar]
24. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. Jama. 2010;304(20):2253-62. [view at publisher] [DOI] [Google Scholar]
25. Bello AI, Owusu-Boakye E, Adegoke BO, Adjei DN. Effects of aerobic exercise on selected physiological parameters and quality of life in patients with type 2 diabetes mellitus. International journal of general medicine. 2011;4:723. [view at publisher] [DOI] [Google Scholar]
26. Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clinical Diabetes. 2004;22(4):169-72. [view at publisher] [DOI] [Google Scholar]
27. Misra A, Alappan NK, Vikram NK, Goel K, Gupta N, Mittal K, et al. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes care. 2008;31(7):1282-7. [view at publisher] [DOI] [Google Scholar]
28. Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Archives of physical medicine and rehabilitation. 2005;86(8):1527-33. [view at publisher] [DOI] [Google Scholar]
29. Shenoy S, Arora E, Jaspal S. Effects of progressive resistance training and aerobic exercise on type 2 diabetics in Indian population. Int J Diabetes Metab. 2009;17(1):27-30. [Google Scholar]
30. Tokmakidis SP, Zois CE, Volaklis KA, Kotsa K, Touvra A-M. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. European journal of applied physiology. 2004;92(4-5):437-42. [view at publisher] [DOI] [Google Scholar]
31. Rahbar S, Naimi SS, Soltani AR, Rahimi A, Akbarzadeh Baghban A, Rashedi V, et al. Improvement in biochemical parameters in patients with type 2 diabetes after twenty-four sessions of aerobic exercise: A randomized controlled trial. Iranian Red Crescent Medical Journal. 2017;19(7). [view at publisher] [DOI] [Google Scholar]
32. Zhang Q-J, Li Q-X, Zhang H-F, Zhang K-R, Guo W-Y, Wang H-C, et al. Swim training sensitizes myocardial response to insulin: role of Akt-dependent eNOS activation. Cardiovascular research. 2007;75(2):369-80. [view at publisher] [DOI] [Google Scholar]
33. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes, metabolic syndrome and obesity: targets and therapy. 2014;7:587. [view at publisher] [DOI] [Google Scholar]
34. Ersoy C, Imamoglu S, Budak F, Tuncel E, Erturk E, Oral B. Effect of amlodipine on insulin resistance & tumor necrosis factor-alpha levels in hypertensive obese type 2 diabetic patients. Indian J Med Res. 2004;120(5):481-8. [Google Scholar]
35. Motahari-Tabari N, Shirvani MA, Shirzad-e-Ahoodashty M, Yousefi-Abdolmaleki E, Teimourzadeh M. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: a randomized clinical trial. Global journal of health science. 2015;7(1):115. [view at publisher] [DOI] [Google Scholar]
36. Detection NCEPEPo, Adults ToHBCi. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): National Cholesterol Education Program, National Heart, Lung, and Blood; 2002. [view at publisher] [DOI]
37. Kizilci S. Physical Activity Level and Related Factors of Diabetic Adults According to Gender. International Journal of Caring Sciences. 2017;10(3):1478-89. [Google Scholar]
38. Olson TP, Dengel D, Leon A, Schmitz K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. International journal of obesity. 2007;31(6):996-1003. [view at publisher] [DOI] [Google Scholar]
39. Almeida MB, Araújo CGS. Effects of aerobic training on heart rate. Revista Brasileira de Medicina do Esporte. 2003;9(2):113-20. [view at publisher] [DOI] [Google Scholar]
40. Nayebifar S, Afzalpour ME, Kazemi T, Abtahi Eivary SH, Mogharnasi M. Changes in blood pressure, body composition, and Vo2max after 10 weeks of high intense interval training and ginger consumption in overweight women. Qom University of Medical Sciences Journal. 2017;11(6):19-27. [view at publisher] [Google Scholar]
41. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes care. 2010;33(12):e147-e67. [view at publisher] [DOI] [Google Scholar]
42. Baharloo S, Taghian F, Hedayati M. Effects of aerobic exercise on C-reactive protein and lipid profile in subclinical hypothyroidism among overweight obese women. Pathobiology Research. 2014;17(1):91-102. [view at publisher] [Google Scholar]
43. Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. New England Journal of Medicine. 2002;347(19):1483-92. [view at publisher] [DOI] [Google Scholar]
44. King D, Brughelli M, Hume P, Gissane C. Assessment, management and knowledge of sport-related concussion: systematic review. Sports medicine. 2014;44(4):449-71. [view at publisher] [DOI] [Google Scholar]
45. Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2. [Google Scholar]
46. Basa AL, Garber AJ. Cardiovascular disease and diabetes: Modifying risk factors other than glucose control. Ochsner Journal. 2001;3(3):132-7. [view at publisher] [Google Scholar]
47. Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundamental & clinical pharmacology. 2009;23(6):681-5. [view at publisher] [DOI] [Google Scholar]
48. Esfarjani F, Rashidi F, Marandi SM. The effect of aerobic exercise on blood glucose, Lipid Profile and Apo. Journal of Ardabil University of Medical Sciences. 2013;13(2):132-41. [view at publisher] [Google Scholar]
49. Sato Y. Overview of Exercise Prescription for Diabetes Patients and Its Application in Japan. Journal of Science in Sport and Exercise. 2020:1-10. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






