Volume 11, Issue 3 (12-2023)
Jorjani Biomed J 2023, 11(3): 1-5 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fallahzadeh V, Taghian F, Jalali Dehkordi K. Exploring the effects of aerobic exercise combined with chitosan nanoparticle-encapsulated ginger on miRNA-214 and SERCA2a in isoproterenol-induced myocardial infarction in rats. Jorjani Biomed J 2023; 11 (3) :1-5
URL: http://goums.ac.ir/jorjanijournal/article-1-984-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-984-en.html
1- Department of Sports Physiology, Faculty of Sports Sciences, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran
2- Department of Sports Physiology, Faculty of Sports Sciences, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran ,ft.taghian@gmail.com
2- Department of Sports Physiology, Faculty of Sports Sciences, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran ,
Keywords: Exercise, Ginger extract, Myocardial infarction, Nanoparticles, Chitosan, SERCA2a, miRNA-214
Full-Text [PDF 588 kb]
(2956 Downloads)
| Abstract (HTML) (10219 Views)
Full-Text: (1809 Views)
Introduction
Cardiovascular disease (CVD) is a common disease worldwide that causes a high mortality rate due to its high prevalence (1). Studies have indicated that the growing population with coronary artery disease in 2008 was about 17 million people, which is expected to reach 23 million in 2030. There are several factors involved in the development of CVD, including high blood pressure, bad lifestyle, poor nutrition, and high stress (2, 3).
Myocardial infarction (MI) is a condition that occurs due to interruption of coronary artery blood flow that inhibits myocardial nutritional needs and oxygen (4). In myocardial infarction, oxidative stress also increases (5). Oxidative stress leads to changes in the structure of phospholipids and proteins, and this action causes lipid peroxidation and thiol group oxidation (5). Oxidative stress may be caused by a defect in the sarcoplasmic reticulum pump Ca2+ - ATPase (SERCA), thereby inhibiting the movement of calcium from the sarcophagus to the cytoplasm of cardiomyocytes (6, 7). An increase in Ca2+ during ischemia converts xanthine dehydrogenase into xanthan oxidase and, consequently, increases O2 production (8, 9). Apart from these changes, MI also affects heart hormones such as natriuretic peptides (ANP), which reportedly suppresses the renin-angiotensin-aldosterone system, endothelin synthesis, and sympathetic nerve activity (10, 11). Treatment with atrial natriuretic peptide (ANP) has been shown to result in a relative reduction of approximately 15% in infarct size and a 2.2% absolute increase in left ventricular ejection fraction, so an increase in ANP synthesis in the early stage of MI is still unclear. Various cellular factors are also required to protect the heart cell, including the miRNA family.
MicroRNAs (miRNAs) are a class of unencoded single-stranded ribonucleic acids (RNAs) that are involved in the regulation of almost all major cellular functions, including development, differentiation, cell proliferation, and apoptosis (12). Recent studies have shown that miRNA-21 is involved in ischemic disease and has a protective effect on heart function (13). Overexpression of miRNA-494 has also been reported to protect against ischemia/reperfusion-induced heart damage, whereas miRNA-320 elevation was harmful (14). miRNA-214 was initially investigated due to its role in apoptosis (15). It has also been shown that miRNA-214 protects against H2O2-induced myocyte damage (16). However, changes in miRNA-214 with antioxidant boosters such as exercise and the use of nano-antioxidant supplements such as nano ginger are unknown.
In MI, there is a disturbance in the balance of oxidative stress and the level of antioxidants in the body. Therefore, the use of antioxidants can minimize the cellular destruction of the heart. Ginger [Zingiberceinale roscoe (Zingiberaceae)] is one of the most famous spices in the world and has been used throughout history for its health benefits (17). A study on the cardiovascular effects of ginger has reported that ginger extract can regulate the amount of superoxide and hydroxyl anion radicals since it can have antioxidant properties (18). Ginger extract is a potent inhibitor of LDL lipoprotein peroxidation and platelet aggregation induced by arachidonic acid and adenosine diphosphate (19). It has been shown that the use of this supplement can also have beneficial effects on the heart tissue. However, for better effect, this supplement can be used in the nanoform.
Applying the knowledge of encapsulation of drugs and anti-cancer substances has been considered in the treatment of various cancers (20). One of the benefits of encapsulation is that it prevents the drug from inducing damage before reaching the target tissue. Chitosan is widely used in pharmaceutical systems due to its non-toxic properties, biocompatibility, biodegradability, and mucosal adhesion (21). Chitosan forms hydrogel particles from a large scale to nanometers under relatively mild gel formation conditions and can, therefore, be used to confine biologically active compounds.
Hydrogels are widely recognized as crosslinked hydrophilic polymer-based systems that can absorb and retain substantial quantities of water without dissolution (22). Consequently, they are extensively utilized as the predominant form of scaffolds (23). Chitosan nanoparticles have been recognized as viable platforms for delivering cardioprotective medicines in the treatment of myocardial ischemia-reperfusion injury (22). As reported by previous research, chitosan nanoparticles administered intravenously exhibit no discernible accumulation in the intact myocardium, irrespective of the ζ-potential. However, Hwang et al. have provided empirical support for the retention of chitosan hydrogel nanoparticles in the damaged heart in vivo (24).
Research has demonstrated that in a sample of human subjects, engaging in exercise training following myocardial infarction (MI) yields beneficial outcomes regarding left ventricular remodeling (25). Furthermore, it leads to improvements in left ventricular function, injection fraction, and diastolic capacity (26, 27). Furthermore, it has been observed that exercise training in animal specimens decreases the collagen content, enhances intracellular calcium ion (Ca2+) handling and calcium sensitivity, and eventually improves the contraction of cardiomyocytes (28, 29)
Regular exercise is an effective factor in controlling heart damage after MI (30). Since regular exercise could increase the antioxidant capacity, it seems that along with the nano ginger supplement, it might have good effects and prove a proper treatment modality for MI. Therefore, in this study, we considered the effects of aerobic exercise with ginger loaded into chitosan nanoparticles on miRNA-214, SERCA2a, atrial natriuretic peptide, and cardiac fibrosis change after isoproterenol-induced myocardial infarction in rats.
Methods
Animals and designing the study groups
All animal studies were performed in accordance with the standard ethical instructions for working with laboratory animals approved by the Azad University of Isfahan (Khorasgan, Iran) and after receiving the code of ethics IR.IAU.KHUISF.REC 1399.041. The rats were offered unrestricted access to food and water throughout the study. Thirty male Wistar rats (8 weeks old) were purchased from Pasteur Institute, Tehran, Iran. The rats were acclimated at the animal laboratory of the department, equipped with all required environmental conditions, including suitable temperature (22 ± 2 °C), 30-70% humidity, and regular 12-hour light/dark cycles. During the study phase, the rats were treated according to standard ethical instructions described for working with laboratory animals (Islamic Azad University, Isfahan, Khorasgan, Iran). The rats were randomly divided into 6 groups (n=5): isoproterenol (Isop), isoproterenol + chitosan nanoparticles (Isop + CNPs), isoproterenol + ginger extract loaded into chitosan nanoparticles (Isop + GE- CNPs), isoproterenol + aerobic exercise (Isop + AE), isoproterenol + ginger extract loaded into chitosan nanoparticles + aerobic exercise (Isop + GE- CNPs + AE), and control (normal group). Following the final training session, the rats were euthanized utilizing xylazine and ketamine anesthesia. Subsequently, the samples were meticulously segregated and preserved for subsequent analyses.
Myocardial infarction-induced models
Isoproterenol was purchased from Sigma-Aldrich (St. Louis, USA). Isop solution at a dose of 85 mg/kg was subcutaneously injected into rats for 2 consecutive days, at a 24-hour interval, to induce the myocardial infarction (MI) rat models (31). After the Isop injection, 5 rats died and were replaced the next day.
Ginger extract preparation
The rhizomes of the ginger plant were rinsed with water and cut into small pieces. The rhizome pieces were then left in the oven for 24 hours to dry. The dried rhizomes were ground into powder form and dissolved in a hydroalcoholic solution (50% ethanol and 50% water). Thereafter, the resulting solution was subjected to the Soxhlet extraction method for 6 hours and then concentrated using a rotary evaporator at 45 °C for 45-50 min. Finally, the resulting chocolate ginger extract was covered with aluminum foil and stored at -20 °C until used. In this study, the ginger extract encapsulated using chitosan nanoparticles at a dose of 500 mg/kg body weight was used for the treatment of MI rat models (32).
Preparation of chitosan nanoparticles
Chitosan nanoparticles (CS-NPs) were fabricated based on the modified ionotropic gelation technique as described by Ibrahim et al., 2017 (33). In brief, chitosan was dissolved in 1% (v/v) acetic acid, and the resulting solution was allowed to stir for 24 hours. The pH was adjusted to 5.5 with 0.01 N NaOH solution. The sodium tripolyphosphate (TPP) aqueous solution was added to the chitosan solution while stirring at room temperature to provide the stability of chitosan nanoparticles. The formed CS-NPs were then precipitated by centrifuging at 8000 × g and 4 °C for 30 min. To remove biopolymers and excessive chemicals, the separated CS NPs were washed with deionized water several times, lyophilized at -60 °C, and stored at 4 °C.
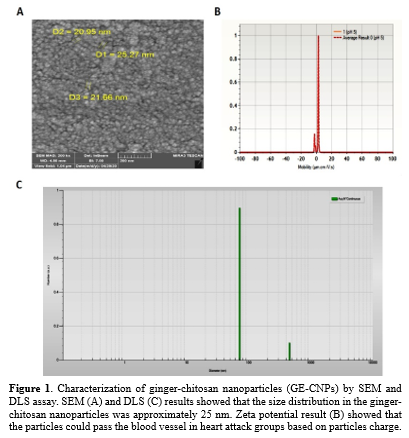
Characterization of ginger-chitosan nanoparticles (GE-CNPs)
The morphological structure of the prepared ginger-chitosan nanoparticles (GE-CNPs) was examined by scanning electron microscopy (SEM, LEO 1430VP, Germany and UK). Moreover, the size distribution and zeta potential of GE-CNPs were determined by dynamic light scattering (DLS) (34) using a Zetasizer Nano ZS (Malvern, UK). To this end, 30 µL of the sample was passed through a 0.2-µm filter. The sample was then transferred into a cuvette and assessed by the Zetaseizer Nano-series instrument.
Exercise training protocol
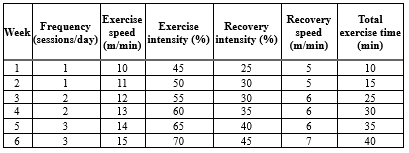
Before MI induction, exercise rats were familiarized with exercise training protocols by running on a treadmill at a speed of 5-8 m/s with no grade for 10 min each day for 5 days. Two days after MI induction, the exercise groups were subjected to the main training protocol for 6 weeks (Table 1). As previously described by Azamian Jazi et al. (2017), in the first week, the training groups began treadmill running at a speed of 10 m/min for 10 min each day. The running and time were gradually increased by 1m/min and 10 min every week and finally enhanced to 15m/min and 60 min a day (including 5 min warm-up and 5 min cool-down) at the end of the sixth week (35).
ELISA Assay
Blood samples were taken from all the groups, and the blood serums were obtained by centrifuging at 300 × g for 10 min to assess the serum activities of creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) in different study groups. The serum creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) levels were detected by ELIZA assay using LDH Assay Kit (Sigma Aldrich, Germany) and Creatine Kinase MB ELISA Kit (Sigma Aldrich, Germany). The procedures were performed according to each kit's instructions.
Quantitative gene expression analyses
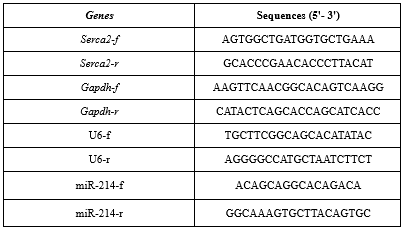
For evaluation of the expression levels of miRNA-214, Serca2a, and ANP genes, the total RNA was isolated from the heart tissues according to the QIAzol lysis reagent protocol (QIAGEN Inc., Valencia, CA), and the cDNAs were synthesized using the cDNA synthesis kit (Thermoscientific, UK). For real-time PCR, miRNA-214, Serca2a, and ANP primers were first designed by the Gene Runner software v. 6.5.52 (Oberlin, San Diego, CA), and the expression levels of the genes of interest were quantitatively measured by the ABI system. The Gapdh gene was used as the housekeeping gene. The PCR thermal program was described as follows: an initial denaturation at 95 °C for 15 min, 40 cycles of 3 steps, including a denaturation step (15 sec at 95 °C), an annealing step (30 sec at 60 °C), and an extension step (30 sec at 72 °C). The relative expression of the genes of interest was calculated by the REST software (versions REST 2009) according to the 2-∆∆Ct method. The sequences of primers are shown in Table 2.
Statistical analyses
All experiments were performed in triplicate, and the data were depicted as the Mean ± SD. Statistical analyses were performed in SPSS v. 23 (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) and Tukey's tests were used to compare the differences among the studied groups. A P-value ≤ 0.05 was statistically significant.
Results
Size and morphology analyses of the prepared ginger-chitosan nanoparticles
The morphological properties of the prepared ginger-chitosan nanoparticles (GE-CNPs) were examined by SEM. As shown in Figure 1A, the GE-CNPs displayed spheroidal shapes and coherent structures. They were homogeneous in size and shape. Furthermore, the DLS analyses indicated that the mean sizes of the prepared Nano-liposomes were in the range of 20-70 nm.
Zeta potential assessment
As illustrated in Figure 1B and C, GE-CNPs showed a zeta potential of 0.2 ± 0.8 mV. The closer the zeta potential value to negative, the more likely the GE-CNP's entry to cells. Our result showed that the GE-CNPs could pass the blood vessels in heart attack groups based on particle charge. The pH of the solution was 5 during measurement under precise temperature control (25 °C) in a 3-mm light path cuvette.
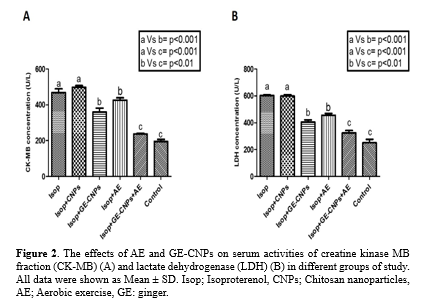
Activity assessment of serum creatine kinase-MB and lactate dehydrogenase
The serum activities of creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) were evaluated using ELIZA assay. The data obtained from all study groups indicated that the serum levels of creatine kinase-MB and lactate dehydrogenase were significantly increased in the Isop group compared to the control (normal) group (P<0.05). After aerobic exercise and treatment with ginger-chitosan nanoparticles or chitosan nanoparticles alone, the serum LDH level was significantly reduced in Isop + AE, Isop + GE-CNPs, and Isop + AE + GE-CNPs groups compared to the Isop group. The serum level of creatine kinase-MB was also significantly decreased in Isop + GE-CNPs and Isop + AE + GE-CNPs groups compared to the Isop group. The lowest activity of both LDH and creatine kinase-MB was observed in the Isop + AE + GE-CNPs group (Figure 2).
Discussion
Lifestyle modification and regular exercise lead to positive physiological cellular adaptations that are suitable for reducing cardiovascular risk factors (36, 37). Aerobic exercise can reduce fibrosis and improve cardiovascular function by strengthening the heart muscle (38). The use of some antioxidant herbal supplements seems to increase the positive effects of exercise training on cardiomyocytes. Therefore, this study aimed to investigate the effect of aerobic exercise along with an herbal ginger supplement (in the nanoform) on improving cardiac tissue, miRNA-214, SERCA2a, and ANP in male Wistar rats with myocardial infarction.
The results of the present study showed that the Isop and Isop + CNPs (Sham) groups had the highest increase in collagen deposition (P <0.05). However, Isop + GE-CNPs, Isop + AE, and Isop + GE-CNPs + AE groups showed a significant reduction in cardiac tissue damage (collagen deposition). Examination of cellular and hormonal variables also showed that Isop + GE-CNPs and Isop + GE-CNPS + AE groups showed a significant increase in miRNA-214 and ANP gene expression, while SERCA2a mRNA significantly increased only in the combination therapy group (Isop+ GE-CNPS + AE). Regarding the effect of ginger on heart tissue, it can be said that the positive effects of ginger on the cardiovascular system have been proven (39, 40). The results of the present study also showed that consumption of ginger, especially in the nano form, improves the structure of heart tissue and reduces collagen deposition. Consistent with the results of the present study, there is evidence that ginger extract may have a positive effect on heart tissue damage (41). Fibrosis and inflammation of the heart cells can be a feature of cardiovascular diseases, such as MI, which decreases the contractile force of the heart muscle. Collagen and fibroblasts change the structure of the heart after fibrosis and cause impaired cardiac function (42). It seems that aerobic exercise with strengthening heart muscles reduces tissue damage caused by MI. In the present study, the greatest reduction in collagen deposition was related to the combination therapy (nano-ginger supplement with aerobic exercise). Consumption of nano-ginger supplement with aerobic exercise seems to be effective in increasing the effects of exercise on heart tissue, including a reduction in cardiac apoptosis and cardiac fibrosis, increased PI3K activity, improvement in calcium in cardiac muscle, enhancement of endothelial function as a result of increased nitric oxide production, increased parasympathetic tone, and dramatic improvements in antioxidant defense (43, 44).
Regarding the increase in miRNA214, it seems that this increase also has positive and cardio-protective effects in groups consuming nano-ginger. Consistent with the results of the present study, Yang et al. (2015) showed that overexpression of miR-214 significantly improved left ventricular (LV) hemodynamic function and LV regeneration in the AMI model rats. The researchers suggested that a possible mediating mechanism of miR-214 was related to the suppression of myocyte apoptosis by suppression of PTEN (45). In the present study, although the changes in apoptosis were not evaluated, it seems that the use of increasing oxidative capacity due to nano-ginger supplementation along with aerobic exercise is effective in inhibiting cardiac cell apoptosis, which needs further investigation because the miRNA 214 maight reduce apoptosis which lead to a new treatment strategy.
Calcium changes and calcium ion exchange can also affect cardiac function after MI. Based on the results of the present study, combination therapy (nano-ginger and aerobic exercise) significantly increased SERCA2a levels. However, aerobic exercise alone could not improve post-MI SERCA2a levels. Contrary to the results of the present study, Bo et al. (2018) showed that intense intermittent exercise with increased NRG1 activates the NRG1/SERCA2a signaling pathway, which improves cardiac function after myocardial infarction (46). It seems that the differences in the type (interval vs. continuous) and duration of exercise (6 weeks in the present study vs. 8 weeks in Bo et al.'s research) are among the reasons for the difference in the expression of SERCA2a in the present study and the study of Bo et al. However, taking nano-supplementation along with aerobic exercise caused significant changes in SERCA2a after MI. It seems that taking this nano-supplement can be better adapted to aerobic exercise in less time.
The expression of miR-214 is dysregulated in ischemia injury and heart failure (47). However, its precise involvement in these pathological conditions remains unclear. Given that the sodium/calcium exchanger 1 (NCX) gene has been confirmed as a target for miRNA-214, it has been proposed that dysregulated levels of miRNA-214 might serve as a mechanism for the downregulation of NCX in a pathological state. However, an increase in NCX was identified in the S-INF group, but it was not found to be associated with miRNA-214. One possible explanation for the elevated activity of the sodium-calcium exchanger (NCX) in the sinoatrial node (S-INF) is that, under certain pathological conditions, the NCX might operate in reverse mode, thereby facilitating the influx of calcium ions (Ca2+) into the cell. This influx of Ca2+ can trigger a phenomenon known as Ca2+-induced Ca2+ release from the sarcoplasmic reticulum, resulting in an excessive accumulation of Ca2+ and subsequent impairment of cardiac function (45-47). In contrast, the T-INF group exhibits a suppression of miRNA-214, a molecule that has been linked to the production of NCX in the S-SHAM group. This effect is consistent with the established mechanism by which ET mitigates Ca2+ excess (28).
Moreover, in the present study, the amount of ANP mRNA in the groups consuming nano-ginger and the combined group of nano-ginger and aerobic exercise showed a significant increase compared to the MI group. Atrial natriuretic peptide (ANP) is a member of the natriuretic peptide family, which exerts its protective functions on the heart not only as a circulating hormone but also as a paracrine hormone. This factor also reduces the size of infarction so incremental changes in this factor in nano-ginger groups and aerobic exercise can indicate the protective effect of this therapeutic modality.
As a limitation, we did not assess the gain and loss of function of miRNA-214 in the condition of myocardial infarction. Moreover, we did not use the antagonism and antagomir of the miRNA-214. In addition, different doses of chitosan nanoparticle-encapsulated ginger were not compared in this study.
Furthermore, the different intensity, duration, and repetition of aerobic exercise training were not evaluated.
Conclusion
It seems that the use of new technology in medicinal plants, such as nano-ginger, along with exercise training, can have a better protective effect on the cardiovascular system. According to the results of the present study, the simultaneous application of nano-ginger with aerobic exercise in MI model rats resulted in positive regulation of miR-214, SERCA2a, and cardiac ANP, which also improved the structure of the cardiac tissue. However, more studies are required on human samples.
Acknowledgement
No acknowledgment
Funding sources
No funding
Ethical statement
All animal studies were performed in accordance with the standard ethical instructions for working with laboratory animals approved by the Azad University of Isfahan (Khorasgan, Iran) and after receiving the code of ethics (IR.IAU.KHUISF.REC 1399.041).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
VF carried out the experiments and drafted the manuscript. FT participated in this study as a supervisor and contributed to study design, conceptualization, data analysis and validation, and revising the manuscript. KJD participated in data validation and revising the manuscript. All the authors read and approved the final manuscript.
Cardiovascular disease (CVD) is a common disease worldwide that causes a high mortality rate due to its high prevalence (1). Studies have indicated that the growing population with coronary artery disease in 2008 was about 17 million people, which is expected to reach 23 million in 2030. There are several factors involved in the development of CVD, including high blood pressure, bad lifestyle, poor nutrition, and high stress (2, 3).
Myocardial infarction (MI) is a condition that occurs due to interruption of coronary artery blood flow that inhibits myocardial nutritional needs and oxygen (4). In myocardial infarction, oxidative stress also increases (5). Oxidative stress leads to changes in the structure of phospholipids and proteins, and this action causes lipid peroxidation and thiol group oxidation (5). Oxidative stress may be caused by a defect in the sarcoplasmic reticulum pump Ca2+ - ATPase (SERCA), thereby inhibiting the movement of calcium from the sarcophagus to the cytoplasm of cardiomyocytes (6, 7). An increase in Ca2+ during ischemia converts xanthine dehydrogenase into xanthan oxidase and, consequently, increases O2 production (8, 9). Apart from these changes, MI also affects heart hormones such as natriuretic peptides (ANP), which reportedly suppresses the renin-angiotensin-aldosterone system, endothelin synthesis, and sympathetic nerve activity (10, 11). Treatment with atrial natriuretic peptide (ANP) has been shown to result in a relative reduction of approximately 15% in infarct size and a 2.2% absolute increase in left ventricular ejection fraction, so an increase in ANP synthesis in the early stage of MI is still unclear. Various cellular factors are also required to protect the heart cell, including the miRNA family.
MicroRNAs (miRNAs) are a class of unencoded single-stranded ribonucleic acids (RNAs) that are involved in the regulation of almost all major cellular functions, including development, differentiation, cell proliferation, and apoptosis (12). Recent studies have shown that miRNA-21 is involved in ischemic disease and has a protective effect on heart function (13). Overexpression of miRNA-494 has also been reported to protect against ischemia/reperfusion-induced heart damage, whereas miRNA-320 elevation was harmful (14). miRNA-214 was initially investigated due to its role in apoptosis (15). It has also been shown that miRNA-214 protects against H2O2-induced myocyte damage (16). However, changes in miRNA-214 with antioxidant boosters such as exercise and the use of nano-antioxidant supplements such as nano ginger are unknown.
In MI, there is a disturbance in the balance of oxidative stress and the level of antioxidants in the body. Therefore, the use of antioxidants can minimize the cellular destruction of the heart. Ginger [Zingiberceinale roscoe (Zingiberaceae)] is one of the most famous spices in the world and has been used throughout history for its health benefits (17). A study on the cardiovascular effects of ginger has reported that ginger extract can regulate the amount of superoxide and hydroxyl anion radicals since it can have antioxidant properties (18). Ginger extract is a potent inhibitor of LDL lipoprotein peroxidation and platelet aggregation induced by arachidonic acid and adenosine diphosphate (19). It has been shown that the use of this supplement can also have beneficial effects on the heart tissue. However, for better effect, this supplement can be used in the nanoform.
Applying the knowledge of encapsulation of drugs and anti-cancer substances has been considered in the treatment of various cancers (20). One of the benefits of encapsulation is that it prevents the drug from inducing damage before reaching the target tissue. Chitosan is widely used in pharmaceutical systems due to its non-toxic properties, biocompatibility, biodegradability, and mucosal adhesion (21). Chitosan forms hydrogel particles from a large scale to nanometers under relatively mild gel formation conditions and can, therefore, be used to confine biologically active compounds.
Hydrogels are widely recognized as crosslinked hydrophilic polymer-based systems that can absorb and retain substantial quantities of water without dissolution (22). Consequently, they are extensively utilized as the predominant form of scaffolds (23). Chitosan nanoparticles have been recognized as viable platforms for delivering cardioprotective medicines in the treatment of myocardial ischemia-reperfusion injury (22). As reported by previous research, chitosan nanoparticles administered intravenously exhibit no discernible accumulation in the intact myocardium, irrespective of the ζ-potential. However, Hwang et al. have provided empirical support for the retention of chitosan hydrogel nanoparticles in the damaged heart in vivo (24).
Research has demonstrated that in a sample of human subjects, engaging in exercise training following myocardial infarction (MI) yields beneficial outcomes regarding left ventricular remodeling (25). Furthermore, it leads to improvements in left ventricular function, injection fraction, and diastolic capacity (26, 27). Furthermore, it has been observed that exercise training in animal specimens decreases the collagen content, enhances intracellular calcium ion (Ca2+) handling and calcium sensitivity, and eventually improves the contraction of cardiomyocytes (28, 29)
Regular exercise is an effective factor in controlling heart damage after MI (30). Since regular exercise could increase the antioxidant capacity, it seems that along with the nano ginger supplement, it might have good effects and prove a proper treatment modality for MI. Therefore, in this study, we considered the effects of aerobic exercise with ginger loaded into chitosan nanoparticles on miRNA-214, SERCA2a, atrial natriuretic peptide, and cardiac fibrosis change after isoproterenol-induced myocardial infarction in rats.
Methods
Animals and designing the study groups
All animal studies were performed in accordance with the standard ethical instructions for working with laboratory animals approved by the Azad University of Isfahan (Khorasgan, Iran) and after receiving the code of ethics IR.IAU.KHUISF.REC 1399.041. The rats were offered unrestricted access to food and water throughout the study. Thirty male Wistar rats (8 weeks old) were purchased from Pasteur Institute, Tehran, Iran. The rats were acclimated at the animal laboratory of the department, equipped with all required environmental conditions, including suitable temperature (22 ± 2 °C), 30-70% humidity, and regular 12-hour light/dark cycles. During the study phase, the rats were treated according to standard ethical instructions described for working with laboratory animals (Islamic Azad University, Isfahan, Khorasgan, Iran). The rats were randomly divided into 6 groups (n=5): isoproterenol (Isop), isoproterenol + chitosan nanoparticles (Isop + CNPs), isoproterenol + ginger extract loaded into chitosan nanoparticles (Isop + GE- CNPs), isoproterenol + aerobic exercise (Isop + AE), isoproterenol + ginger extract loaded into chitosan nanoparticles + aerobic exercise (Isop + GE- CNPs + AE), and control (normal group). Following the final training session, the rats were euthanized utilizing xylazine and ketamine anesthesia. Subsequently, the samples were meticulously segregated and preserved for subsequent analyses.
Myocardial infarction-induced models
Isoproterenol was purchased from Sigma-Aldrich (St. Louis, USA). Isop solution at a dose of 85 mg/kg was subcutaneously injected into rats for 2 consecutive days, at a 24-hour interval, to induce the myocardial infarction (MI) rat models (31). After the Isop injection, 5 rats died and were replaced the next day.
Ginger extract preparation
The rhizomes of the ginger plant were rinsed with water and cut into small pieces. The rhizome pieces were then left in the oven for 24 hours to dry. The dried rhizomes were ground into powder form and dissolved in a hydroalcoholic solution (50% ethanol and 50% water). Thereafter, the resulting solution was subjected to the Soxhlet extraction method for 6 hours and then concentrated using a rotary evaporator at 45 °C for 45-50 min. Finally, the resulting chocolate ginger extract was covered with aluminum foil and stored at -20 °C until used. In this study, the ginger extract encapsulated using chitosan nanoparticles at a dose of 500 mg/kg body weight was used for the treatment of MI rat models (32).
Preparation of chitosan nanoparticles
Chitosan nanoparticles (CS-NPs) were fabricated based on the modified ionotropic gelation technique as described by Ibrahim et al., 2017 (33). In brief, chitosan was dissolved in 1% (v/v) acetic acid, and the resulting solution was allowed to stir for 24 hours. The pH was adjusted to 5.5 with 0.01 N NaOH solution. The sodium tripolyphosphate (TPP) aqueous solution was added to the chitosan solution while stirring at room temperature to provide the stability of chitosan nanoparticles. The formed CS-NPs were then precipitated by centrifuging at 8000 × g and 4 °C for 30 min. To remove biopolymers and excessive chemicals, the separated CS NPs were washed with deionized water several times, lyophilized at -60 °C, and stored at 4 °C.
Characterization of ginger-chitosan nanoparticles (GE-CNPs)
The morphological structure of the prepared ginger-chitosan nanoparticles (GE-CNPs) was examined by scanning electron microscopy (SEM, LEO 1430VP, Germany and UK). Moreover, the size distribution and zeta potential of GE-CNPs were determined by dynamic light scattering (DLS) (34) using a Zetasizer Nano ZS (Malvern, UK). To this end, 30 µL of the sample was passed through a 0.2-µm filter. The sample was then transferred into a cuvette and assessed by the Zetaseizer Nano-series instrument.
Exercise training protocol
Before MI induction, exercise rats were familiarized with exercise training protocols by running on a treadmill at a speed of 5-8 m/s with no grade for 10 min each day for 5 days. Two days after MI induction, the exercise groups were subjected to the main training protocol for 6 weeks (Table 1). As previously described by Azamian Jazi et al. (2017), in the first week, the training groups began treadmill running at a speed of 10 m/min for 10 min each day. The running and time were gradually increased by 1m/min and 10 min every week and finally enhanced to 15m/min and 60 min a day (including 5 min warm-up and 5 min cool-down) at the end of the sixth week (35).
|
Table 1. Exercise training protocol
 |
Blood samples were taken from all the groups, and the blood serums were obtained by centrifuging at 300 × g for 10 min to assess the serum activities of creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) in different study groups. The serum creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) levels were detected by ELIZA assay using LDH Assay Kit (Sigma Aldrich, Germany) and Creatine Kinase MB ELISA Kit (Sigma Aldrich, Germany). The procedures were performed according to each kit's instructions.
Quantitative gene expression analyses
For evaluation of the expression levels of miRNA-214, Serca2a, and ANP genes, the total RNA was isolated from the heart tissues according to the QIAzol lysis reagent protocol (QIAGEN Inc., Valencia, CA), and the cDNAs were synthesized using the cDNA synthesis kit (Thermoscientific, UK). For real-time PCR, miRNA-214, Serca2a, and ANP primers were first designed by the Gene Runner software v. 6.5.52 (Oberlin, San Diego, CA), and the expression levels of the genes of interest were quantitatively measured by the ABI system. The Gapdh gene was used as the housekeeping gene. The PCR thermal program was described as follows: an initial denaturation at 95 °C for 15 min, 40 cycles of 3 steps, including a denaturation step (15 sec at 95 °C), an annealing step (30 sec at 60 °C), and an extension step (30 sec at 72 °C). The relative expression of the genes of interest was calculated by the REST software (versions REST 2009) according to the 2-∆∆Ct method. The sequences of primers are shown in Table 2.
|
Table 2. The sequences of primers used for real-time polymerase chain reaction
 |
All experiments were performed in triplicate, and the data were depicted as the Mean ± SD. Statistical analyses were performed in SPSS v. 23 (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) and Tukey's tests were used to compare the differences among the studied groups. A P-value ≤ 0.05 was statistically significant.
Results
Size and morphology analyses of the prepared ginger-chitosan nanoparticles
The morphological properties of the prepared ginger-chitosan nanoparticles (GE-CNPs) were examined by SEM. As shown in Figure 1A, the GE-CNPs displayed spheroidal shapes and coherent structures. They were homogeneous in size and shape. Furthermore, the DLS analyses indicated that the mean sizes of the prepared Nano-liposomes were in the range of 20-70 nm.
Zeta potential assessment
As illustrated in Figure 1B and C, GE-CNPs showed a zeta potential of 0.2 ± 0.8 mV. The closer the zeta potential value to negative, the more likely the GE-CNP's entry to cells. Our result showed that the GE-CNPs could pass the blood vessels in heart attack groups based on particle charge. The pH of the solution was 5 during measurement under precise temperature control (25 °C) in a 3-mm light path cuvette.

Activity assessment of serum creatine kinase-MB and lactate dehydrogenase
The serum activities of creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) were evaluated using ELIZA assay. The data obtained from all study groups indicated that the serum levels of creatine kinase-MB and lactate dehydrogenase were significantly increased in the Isop group compared to the control (normal) group (P<0.05). After aerobic exercise and treatment with ginger-chitosan nanoparticles or chitosan nanoparticles alone, the serum LDH level was significantly reduced in Isop + AE, Isop + GE-CNPs, and Isop + AE + GE-CNPs groups compared to the Isop group. The serum level of creatine kinase-MB was also significantly decreased in Isop + GE-CNPs and Isop + AE + GE-CNPs groups compared to the Isop group. The lowest activity of both LDH and creatine kinase-MB was observed in the Isop + AE + GE-CNPs group (Figure 2).

Discussion
Lifestyle modification and regular exercise lead to positive physiological cellular adaptations that are suitable for reducing cardiovascular risk factors (36, 37). Aerobic exercise can reduce fibrosis and improve cardiovascular function by strengthening the heart muscle (38). The use of some antioxidant herbal supplements seems to increase the positive effects of exercise training on cardiomyocytes. Therefore, this study aimed to investigate the effect of aerobic exercise along with an herbal ginger supplement (in the nanoform) on improving cardiac tissue, miRNA-214, SERCA2a, and ANP in male Wistar rats with myocardial infarction.
The results of the present study showed that the Isop and Isop + CNPs (Sham) groups had the highest increase in collagen deposition (P <0.05). However, Isop + GE-CNPs, Isop + AE, and Isop + GE-CNPs + AE groups showed a significant reduction in cardiac tissue damage (collagen deposition). Examination of cellular and hormonal variables also showed that Isop + GE-CNPs and Isop + GE-CNPS + AE groups showed a significant increase in miRNA-214 and ANP gene expression, while SERCA2a mRNA significantly increased only in the combination therapy group (Isop+ GE-CNPS + AE). Regarding the effect of ginger on heart tissue, it can be said that the positive effects of ginger on the cardiovascular system have been proven (39, 40). The results of the present study also showed that consumption of ginger, especially in the nano form, improves the structure of heart tissue and reduces collagen deposition. Consistent with the results of the present study, there is evidence that ginger extract may have a positive effect on heart tissue damage (41). Fibrosis and inflammation of the heart cells can be a feature of cardiovascular diseases, such as MI, which decreases the contractile force of the heart muscle. Collagen and fibroblasts change the structure of the heart after fibrosis and cause impaired cardiac function (42). It seems that aerobic exercise with strengthening heart muscles reduces tissue damage caused by MI. In the present study, the greatest reduction in collagen deposition was related to the combination therapy (nano-ginger supplement with aerobic exercise). Consumption of nano-ginger supplement with aerobic exercise seems to be effective in increasing the effects of exercise on heart tissue, including a reduction in cardiac apoptosis and cardiac fibrosis, increased PI3K activity, improvement in calcium in cardiac muscle, enhancement of endothelial function as a result of increased nitric oxide production, increased parasympathetic tone, and dramatic improvements in antioxidant defense (43, 44).
Regarding the increase in miRNA214, it seems that this increase also has positive and cardio-protective effects in groups consuming nano-ginger. Consistent with the results of the present study, Yang et al. (2015) showed that overexpression of miR-214 significantly improved left ventricular (LV) hemodynamic function and LV regeneration in the AMI model rats. The researchers suggested that a possible mediating mechanism of miR-214 was related to the suppression of myocyte apoptosis by suppression of PTEN (45). In the present study, although the changes in apoptosis were not evaluated, it seems that the use of increasing oxidative capacity due to nano-ginger supplementation along with aerobic exercise is effective in inhibiting cardiac cell apoptosis, which needs further investigation because the miRNA 214 maight reduce apoptosis which lead to a new treatment strategy.
Calcium changes and calcium ion exchange can also affect cardiac function after MI. Based on the results of the present study, combination therapy (nano-ginger and aerobic exercise) significantly increased SERCA2a levels. However, aerobic exercise alone could not improve post-MI SERCA2a levels. Contrary to the results of the present study, Bo et al. (2018) showed that intense intermittent exercise with increased NRG1 activates the NRG1/SERCA2a signaling pathway, which improves cardiac function after myocardial infarction (46). It seems that the differences in the type (interval vs. continuous) and duration of exercise (6 weeks in the present study vs. 8 weeks in Bo et al.'s research) are among the reasons for the difference in the expression of SERCA2a in the present study and the study of Bo et al. However, taking nano-supplementation along with aerobic exercise caused significant changes in SERCA2a after MI. It seems that taking this nano-supplement can be better adapted to aerobic exercise in less time.
The expression of miR-214 is dysregulated in ischemia injury and heart failure (47). However, its precise involvement in these pathological conditions remains unclear. Given that the sodium/calcium exchanger 1 (NCX) gene has been confirmed as a target for miRNA-214, it has been proposed that dysregulated levels of miRNA-214 might serve as a mechanism for the downregulation of NCX in a pathological state. However, an increase in NCX was identified in the S-INF group, but it was not found to be associated with miRNA-214. One possible explanation for the elevated activity of the sodium-calcium exchanger (NCX) in the sinoatrial node (S-INF) is that, under certain pathological conditions, the NCX might operate in reverse mode, thereby facilitating the influx of calcium ions (Ca2+) into the cell. This influx of Ca2+ can trigger a phenomenon known as Ca2+-induced Ca2+ release from the sarcoplasmic reticulum, resulting in an excessive accumulation of Ca2+ and subsequent impairment of cardiac function (45-47). In contrast, the T-INF group exhibits a suppression of miRNA-214, a molecule that has been linked to the production of NCX in the S-SHAM group. This effect is consistent with the established mechanism by which ET mitigates Ca2+ excess (28).
Moreover, in the present study, the amount of ANP mRNA in the groups consuming nano-ginger and the combined group of nano-ginger and aerobic exercise showed a significant increase compared to the MI group. Atrial natriuretic peptide (ANP) is a member of the natriuretic peptide family, which exerts its protective functions on the heart not only as a circulating hormone but also as a paracrine hormone. This factor also reduces the size of infarction so incremental changes in this factor in nano-ginger groups and aerobic exercise can indicate the protective effect of this therapeutic modality.
As a limitation, we did not assess the gain and loss of function of miRNA-214 in the condition of myocardial infarction. Moreover, we did not use the antagonism and antagomir of the miRNA-214. In addition, different doses of chitosan nanoparticle-encapsulated ginger were not compared in this study.
Furthermore, the different intensity, duration, and repetition of aerobic exercise training were not evaluated.
Conclusion
It seems that the use of new technology in medicinal plants, such as nano-ginger, along with exercise training, can have a better protective effect on the cardiovascular system. According to the results of the present study, the simultaneous application of nano-ginger with aerobic exercise in MI model rats resulted in positive regulation of miR-214, SERCA2a, and cardiac ANP, which also improved the structure of the cardiac tissue. However, more studies are required on human samples.
Acknowledgement
No acknowledgment
Funding sources
No funding
Ethical statement
All animal studies were performed in accordance with the standard ethical instructions for working with laboratory animals approved by the Azad University of Isfahan (Khorasgan, Iran) and after receiving the code of ethics (IR.IAU.KHUISF.REC 1399.041).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
VF carried out the experiments and drafted the manuscript. FT participated in this study as a supervisor and contributed to study design, conceptualization, data analysis and validation, and revising the manuscript. KJD participated in data validation and revising the manuscript. All the authors read and approved the final manuscript.
Type of Article: Original article |
Subject:
Molecular Sciences
Received: 2023/08/26 | Accepted: 2023/11/4 | Published: 2023/12/19
Received: 2023/08/26 | Accepted: 2023/11/4 | Published: 2023/12/19
References
1. Sarrafzadegan N, Mohammmadifard N. Cardiovascular disease in Iran in the last 40 years: prevalence, mortality, morbidity, challenges and strategies for cardiovascular prevention. Arch Iran Med. 2019;22(4):204-10. [View at Publisher] [PMID] [Google Scholar]
2. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285-92. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Dar T, Radfar A, Abohashem S, Pitman RK, Tawakol A, Osborne MT. Psychosocial stress and cardiovascular disease. Curr Treat Options Cardiovasc Med. 2019;21(5):23. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Sharedalal P, Aronow WS. A review of diagnosis, etiology, assessment, and management of patients with myocardial infarction in the absence of obstructive coronary artery disease. Hosp Pract. 2021;49(1):12-21. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Fuentes E, Moore-Carrasco R, de Andrade Paes AM, Trostchansky A. Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J Cardiovasc Pharmacol Ther. 2019;24(6):509-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Li C, Ma Q, Toan S, Wang J, Zhou H, Liang J. SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways. Redox Biol. 2020;36:101659. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Li Y, Wang X, Lou C. Gastrodin pretreatment impact on sarcoplasmic reticulum calcium transport ATPase (SERCA) and calcium phosphate (PLB) expression in rats with myocardial ischemia reperfusion. Med Sci Monit. 2016;22:3309-15. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. González-Montero J, Brito R, Gajardo AI, Rodrigo R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J Cardiol. 2018;10(9):74-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Lee M-C-i, Velayutham M, Komatsu T, Hille R, Zweier JL. Measurement and characterization of superoxide generation from xanthine dehydrogenase: a redox-regulated pathway of radical generation in ischemic tissues. Biochemistry. 2014;53(41):6615-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2008;29(12):1485-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Chen HH, Martin FL, Gibbons RJ, Schirger JA, Wright RS, Schears RM, et al. Low-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept study. Heart. 2009;95(16):1315-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Nana-Sinkam SP, Croce CM. Non-coding RNAs in cancer initiation and progression and as novel biomarkers. Mol Oncol. 2011;5(6):483-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284(43):29514-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Wang X, Zhu H, Zhang X, Liu Y, Chen J, Medvedovic M, et al. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc Res. 2012;94(2):379-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Wang Z, Cai H, Lin L, Tang M, Cai H. Upregulated expression of microRNA‐214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatric blood & cancer. 2014;61(2):206-10. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Lv G, Shao S, Dong H, Bian X, Yang X, Dong S. MicroRNA‐214 protects cardiac myocytes against H2O2‐induced injury. J Cell Biochem. 2014;115(1):93-101. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Hassanien MA. Ameliorating effects of ginger on isoproterenol-induced acute myocardial infarction in rats and its impact on cardiac nitric oxide. J Microsc Ultrastruct. 2020;8(3):96-103. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Chen G-t, Yuan B, Wang H-x, Qi G-h, Cheng S-j. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int J Biol Macromol. 2019;139:801-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Fuhrman B, Rosenblat M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130(5):1124-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Hofheinz R-D, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16(7):691-707. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Release. 2003;89(2):151-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657-66. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Kazemzadeh-Narbat M, Reid M, Brooks MS-L, Ghanem A. Chitosan nanoparticles as adenosine carriers. J Microencapsul. 2015;32(5):460-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Hwang H, Kwon J, Oh P-S, Lee T-K, Na K-S, Lee C-M, et al. Peptide-loaded nanoparticles and radionuclide imaging for individualized treatment of myocardial ischemia. Radiology. 2014;273(1):160-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Abedpoor N, Taghian F, Hajibabaie F. Physical activity ameliorates the function of organs via adipose tissue in metabolic diseases. Acta Histochem. 2022;124(2):151844. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162(4):571-84. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011(7). [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54(1):162-74. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Yengo C, Zimmerman SD, McCormick RJ, Thomas DP. Exercise training post-MI favorably modifies heart extracellular matrix in the rat. Med Sci Sports Exerc. 2012;44(6):1005-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Zhao D, Sun Y, Tan Y, Zhang Z, Hou Z, Gao C, et al. Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxid Med Cell longev. 2018;2018:4079041. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225(1-2):75-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Rong X, Peng G, Suzuki T, Yang Q, Yamahara J, Li Y. A 35-day gavage safety assessment of ginger in rats. Regul Toxicol Pharmacol. 2009;54(2):118-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Sabry S, Sakr SM, Ibrahim HM. Effect of chitosan nanoparticles on haloperidol drug-induced hepatotoxicity in albino rats: Light and Electron Microscopic study. Journal of Bioscience and Applied Research. 2016;2(12):771-8. [View at Publisher] [DOI] [Google Scholar]
34. Mason G, Wilking J, Meleson K, Chang C, Graves S. Nanoemulsions: Formation, structure, and physical properties. Journal of Physics: Condensed Matter. 2006;18:R635. [View at Publisher] [DOI] [Google Scholar]
35. Azamian Jazi A, Abdi H, Haffezi Ahmadi MR, Cheraghi J. Effect of endurance exercise training on morphological changes in rat heart tissue following experimental myocardial infarction. J Bas Res Med Sci. 2017;4(1):8-16. [View at Publisher] [DOI] [Google Scholar]
36. Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Weterings S, Oppewal A, Hilgenkamp TI. The feasibility of vigorous resistance exercise training in adults with intellectual disabilities with cardiovascular disease risk factors. J Appl Res Intellect Disabil. 2020;33(3):488-95. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Siasos G, Athanasiou D, Terzis G, Stasinaki A, Oikonomou E, Tsitkanou S, et al. Acute effects of different types of aerobic exercise on endothelial function and arterial stiffness. Eur J Prev Cardiol. 2016;23(14):1565-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: a review. Critical reviews in food science and nutrition. 2013;53(7):659-69. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Choi JG, Kim SY, Jeong M, Oh MS. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol Ther. 2018;182:56-69. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on some cardiovascular risk factors in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65(4):515-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
42. Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci. 2017;1398(1):83-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
43. Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108(5):554-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
44. Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095-101. [View at Publisher] [DOI] [PMID] [Google Scholar]
45. Yang X, Qin Y, Shao S, Yu Y, Zhang C, Dong H, et al. MicroRNA-214 inhibits left ventricular remodeling in an acute myocardial infarction rat model by suppressing cellular apoptosis via the phosphatase and tensin homolog (PTEN). Int Heart J. 2016;57(2):247-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
46. Bo W, Li D, Tian Z. PO-144 Intermittent Exercise Activates NRG1-SERCA2a Pathway to Improve Cardiac Function in Myocardial Infarction Rats. Exercise Biochemistry Review. 2018;1(4). [View at Publisher] [DOI] [Google Scholar]
47. Gaudin A, Yemisci M, Eroglu H, Lepetre-Mouelhi S, Turkoglu OF, Dönmez-Demir B, et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol. 2014;9(12):1054-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






