Volume 12, Issue 4 (12-2024)
Jorjani Biomed J 2024, 12(4): 5-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Valikhani S, Avandi S M, Hedayati M. Effect of an eight-week multi-joint circuit resistance training program on adiponectin levels and lipid profiles in overweight women from Absard City. Jorjani Biomed J 2024; 12 (4) :5-8
URL: http://goums.ac.ir/jorjanijournal/article-1-981-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-981-en.html
1- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,m.avandi@semnan.ac.ir
3- Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,
3- Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Iran
Full-Text [PDF 453 kb]
(1444 Downloads)
| Abstract (HTML) (5376 Views)
Discussion
According to the results of the present study, eight weeks of circuit resistance training significantly decreased BW, BMI, fat percentage, LDL, and cholesterol, while significantly increasing adiponectin levels in the training group compared to the control group. However, no significant changes were observed in TG and HDL levels. Consistently, Ricci et al. reported increased adiponectin levels and improved insulin resistance after eight weeks of resistance training (33). According to Zamani et al., eight weeks of resistance training significantly increased adiponectin and testosterone levels in healthy male participants (34). Furthermore, Dehrashid et al. reported a significant increase in adiponectin levels after 12 weeks of circuit resistance training (35). Similarly, Dehrashid et al. observed a significant increase in adiponectin levels following 10 weeks of resistance training. In the mentioned research, the reduction in TG, LDL, and cholesterol was not considered significant (35).
According to the literature, long-term sports activities increase adiponectin levels (36). While all the studies in this regard involve eight weeks of training, the interventions range from high-intensity interval training to endurance training (36). Moreover, the duration of these interventions varies, with the number of weekly sessions ranging from a minimum of three per week (21,37) to a maximum of seven per week (38-40).
In a study conducted by Vardar et al., two training protocols commonly used in community training programs were compared. The proper exercise volume (Six sessions per week) was highlighted as a strength of the exercise protocol in the mentioned study, while the inadequate adiponectin measurement (Gene expression alone without assessing plasma adiponectin levels) was noted as a limitation (41). Studies conducted by Karajbani et al. (2018), Rostamizadeh et al. (2018), Hosseini et al. (2018), Mosaf and Abedi (2018), Ghaleno et al. (2017), Bettiol et al. (2017), Albashi et al. (2017), Piri and Zamani (2016), Nader et al. (2016), Bouri and Piri (2015), and Wang et al. (2015) were similar to the current research in terms of objectives and design. The common positive aspects of these studies included the number and type of participants (Obese and overweight individuals and patients) and the appropriate length of the interventions (8-20 weeks). However, the limitations of these studies included the use of the maximum heart rate index instead of maximum oxygen consumption in most cases, which might have reduced the accuracy of controlling training intensity and resulted in the relatively low volume of training (Three sessions per week).
Adiponectin is a protein hormone secreted by adipocytes that aids in insulin sensitivity and inflammation (18), regulating glucose metabolism in skeletal muscle and adipocytes through the activation of phosphorylated AMP-activated protein kinase and proliferator-activated receptors. Obese individuals have a lower plasma concentration of adiponectin compared to those with normal weight, and a reduction in BW and BFM increases adiponectin levels in the blood (18).
Controlling environmental confounding factors is one of the key strengths of the animal studies conducted by Vardar et al (2018) and Racil et al. (2013). In these studies, less attention was given to the type of diet, which could be considered a limitation. Meanwhile, high training intensity and the use of the maximum oxygen consumption index instead of the maximum heart rate increased the accuracy of these studies. The relatively small number of participants and training sessions might have also affected the results of the aforementioned studies (41,42).
Voss et al. (2016) conducted research on individuals with athletic bodies, reporting that exercise background could affect the adiponectin response to sports activities (43). Therefore, performing similar studies on non-athletic individuals could yield interesting results. In another study, Saunders et al. (2012) evaluated the effect of moderate-intensity running (Close to the anaerobic threshold) on obese individuals. One of the strengths of the mentioned study was its appropriate number of participants (n=38) and their condition (Obesity). Meanwhile, their exercise protocol (i.e., running) burns a considerable number of calories, which might have altered the adiponectin levels of the subjects more significantly. In addition, VO2max is considered a more accurate index for controlling exercise intensity (44).
Given the involvement of large muscles, multi-joint bodyweight resistance exercises increase energy consumption, which is essential for overweight individuals (45). Therefore, it seems that eight weeks of circuit training could increase adiponectin levels while saving time. Furthermore, these exercises could decrease LDL, cholesterol, body fat percentage, and weight by increasing energy consumption. It is suggested that multi-joint and aerobic exercises be incorporated into circuit training protocols for overweight individuals.
Conclusion
According to the findings of this study, an 8-week circuit resistance training program can be effective in improving adiponectin levels and lipid profile in obese women.
Acknowledgement
The authors of this article would like to express their gratitude to Semnan University and all the subjects who participated in this study.
Funding sources
Not applicable.
Ethical statement
The study protocol was approved by the Ethics Committee of the Sports Sciences Research Institute (Code: IR.SSRC.REC.1398.114).
Conflicts of interest
The authors have no conflicts of interest that are directly relevant to the content of this original research paper.
Author contributions
Sara Valikhani contributed to data collection, statistical analysis. Mehdi Hedayati contributed to laboratory experiments. Seyed Mohsen Avandi conceived and supervised the study. All authors read and approved the final manuscript.
Full-Text: (879 Views)
Introduction
Obesity has increased significantly over the past few decades worldwide (1). Weight gain occurs due to an energy imbalance, with the body receiving more energy than needed (2). Adipose tissue dysfunction leads to conditions such as metabolic syndrome, cardiovascular disorders, and cancer in obese individuals (3). In general, metabolic syndrome is defined as the presence of at least three clinical risk factors, including abdominal (Visceral) obesity, hypertension, dyslipidemia, decreased high-density lipoproteins, high triglycerides (TGs), free fatty acids, low-density lipoproteins, and insulin resistance (4).
The adipose tissue was initially regarded as an energy storage organ; however, it has recently been identified as an active endocrine tissue (5). Adipose tissue produces and releases various pro-inflammatory and anti-inflammatory factors, including leptin, adiponectin, visfatin, resistin, and omentin, which function at autocrine, paracrine, and endocrine levels (6). Adiponectin is a protein composed of 244 amino acids and is found in the blood in three different molecular weights: low molecular weight, moderate molecular weight, and high molecular weight (7). High molecular weight is most significantly associated with obesity, cardiovascular diseases, and metabolic syndrome (8). Adiponectin activates adenosine monophosphate-activated protein kinase (AMPK) through the adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1. AMPK is primarily a signaling pathway that ultimately increases beta-oxidation, glucose transporter type 4, cluster of differentiation 36, and glucose and fatty acid intake by cells (8).
Adiponectin exerts its effects through two membrane receptors, AdipoR1 and AdipoR2; the former is primarily found in muscles, and the latter in the liver (9). In addition, adiponectin activates AMPK phosphorylation by binding to AdipoR1, thereby increasing glucose use and fatty acid oxidation in muscles and the liver (10). Adiponectin also activates PGC-1, a cell receptor that facilitates the release of mitochondrial proteins (11). PGC-1 regulates lipid metabolism and long-chain fatty acid oxidation through the expression of several carboxylic acid cycle genes and the mitochondrial fatty acid pathway (12). Furthermore, PGC-1 increases the expression of fibronectin type III domain-containing protein 5 (13). This receptor also stimulates mitochondrial biogenesis and angiogenesis while reducing atrophy.
Adiponectin has antidiabetic properties due to its ability to increase insulin sensitivity (14). Studies indicate decreased adiponectin levels in obese individuals, which lead to increased susceptibility to health conditions such as metabolic syndrome, insulin resistance, and type II diabetes (15). A negative correlation has also been reported between this protein and the body mass index (BMI) (16). Conversely, increased adiponectin levels can significantly reduce total cholesterol, low-density lipoprotein (LDL), and TGs while increasing high-density lipoprotein (HDL) (17).
Esposito et al. (2003) reported that aerobic exercise for two years in obese middle-aged women resulted in a decrease in body weight (BW) along with an increase in plasma adiponectin concentration. Contrary to these positive effects of exercise on adiponectin levels in the blood of obese individuals, Hara et al. (2005) suggested that complex exercise for eight weeks in obese adolescents did not induce changes in plasma adiponectin concentration, and Polak et al. (2006) reported that aerobic exercise for 12 weeks did not increase blood adiponectin concentration in obese individuals (18).
In a study, six weeks of acute aerobic training at an intensity of 40-50% VO2max was reported to have no significant effect on adiponectin (19). Another study assessed the effect of aerobic training with varied intensities on adiponectin, indicating that eight weeks of low-intensity aerobic exercise had no significant effect on adiponectin. Meanwhile, moderate- and high-intensity aerobic training were shown to significantly increase adiponectin. In a related study, one session of aerobic training at an intensity of 65% VO2max significantly reduced adiponectin (20).
In general, regular resistance training has been found to be effective in decreasing body fat in women and increasing adiponectin (21). High-intensity interval training significantly increases serum adiponectin (22). A study in this regard showed an increase in serum adiponectin levels following resistance and aerobic training, which improved inflammation and metabolic syndrome (23). Evidence suggests that regular physical activity also reduces TGs and LDL while increasing HDL (24). The most significant LDL reduction and HDL increase have been reported in individuals performing aerobic training at an intensity of 55-65% VO2max (24). Furthermore, resistance training has been shown to significantly affect the lipid profile. A study indicated a significant decrease in TG and LDL and a significant increase in HDL following resistance training exercises (25).
Recently, combined aerobic and resistance training has been suggested to have a more favorable impact on cardiovascular and general health compared to aerobic and resistance training performed separately (26). Circuit training, which is beneficial to overweight individuals, is a combination of aerobic and resistance training (27). These exercises increase muscle endurance and strength, as well as aerobic function (28). Previous studies have indicated that circuit training is associated with a significant decrease in blood pressure and an increase in muscle tissue, strength, and aerobic capacity (29). Moreover, circuit training has been reported to improve metabolism by increasing oxygen intake (29).
Considering these research findings, the effect of exercise type, intensity, and/or duration on blood adiponectin levels in obese individuals remains controversial. Therefore, the purpose of this study was to investigate the effect of eight weeks of resistance circuit training on plasma adiponectin levels and lipid profiles in overweight women.
Methods
This quasi-experimental research was performed with a pretest-posttest design and a control group. After the call, volunteers were invited to participate in the project through a public announcement. In addition, patients with medical records at the Research Institute were informed about and invited to the project through phone calls. The sample population included overweight women aged 25-45 years. Inclusion criteria were a BMI of 28-40 kg/m2, absence of specific diseases, and no regular sports activity within the past six months. Individuals with hereditary hypolipoproteinemia and other chronic diseases, such as diabetes mellitus, or those who were drug users, were excluded from the study. In total, 30 women with a mean age of 34±4 years, mean height of 165.35±7.5 centimeters, mean weight of 81.17±9.13 kilograms, and mean BMI of 30.17±2.13 kg/m2 were enrolled in the study. The participants were randomly divided into two groups: circuit resistance training (n=15) and control (n=15). The present research was conducted in Absard City in the winter of 2020.
In one session, the subjects were familiarized with the type of the study, its objectives, implementation methods, and possible risks, and informed consent was obtained. Data were collected using the Physical Activity Readiness Questionnaire prior to the research. Notably, 10 participants withdrew from the research for personal reasons. Finally, eight and 10 participants remained in the training and control groups, respectively. The study protocol was approved by the Ethics Committee of the Sports Sciences Research Institute (Code: IR.SSRC.REC.1398.114).
Anthropometric indices, including height, weight, fat percentage, and abdominal circumference, were measured. Height was measured using a tape measure with 0.5 cm precision, and the fat percentage of the participants was calculated using a skinfold caliper, which measures subcutaneous fat based on a three-site method (triceps, pelvis, and thigh) on the right side of the body, after applying the digits obtained by the Jackson and Pollock body density formula (30). Furthermore, maximum strength was measured using an indirect method involving leg and chest press movements to assess lower body and upper body strength, respectively (31).
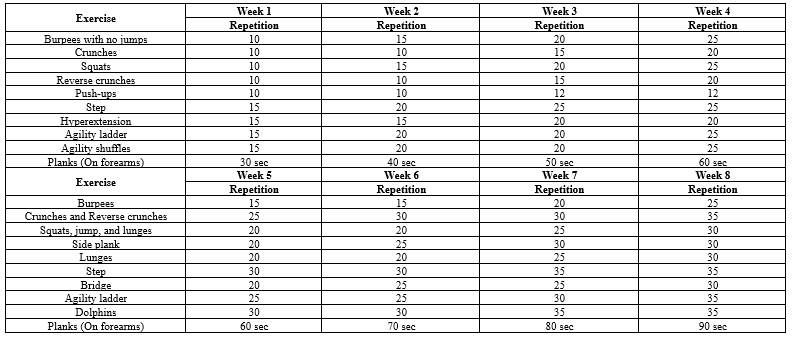
Chemical variables were measured based on blood samples (5 cc) collected from the participants by a specialist at two stages: 48 hours before the first exercise session and 48 hours after the last exercise session. The samples were collected in a laboratory after 8-10 hours of fasting. After centrifuging the blood samples at 2,500 rpm/min, the samples were transferred to the Shahid Beheshti Endocrinology and Metabolism Research Institute in Tehran, Iran, in a special package at a specific temperature. The exercise protocol was performed by the training group in a multifunctional center, with three sessions per week for eight weeks. The control group was assigned no regular physical activity (Table 1).
With regard to the biochemical indices, adiponectin was measured using the ELISA assay with a sensitivity of 0.1 ng/l and an intragroup change coefficient of 7.2%, using the Zellbio GmbH kit (Made in Germany). TGs were measured using a colorimetric enzymatic method with a sensitivity of 1 mg/dl and an intragroup change coefficient of 2.3%, using the Pars test kit. Cholesterol was measured using an enzymatic photometric method with a sensitivity of 3 mg/dl and an intragroup change coefficient of 2.1%, using the Pars test kit. HDL was measured using an enzymatic photometric method with a sensitivity of 1 mg/dl and an intragroup change coefficient of 3.4%, using the Pars test kit. LDL was measured using the Friedewald formula (32).
Data analysis was performed in SPSS version 24 using the Shapiro-Wilk test to determine the normal distribution of data among the groups. In addition, Levene’s test was used to assess the homogeneity of variance between the groups. One-way analysis of variance (ANOVA) and dependent t-test were also applied at the significance level of P-value ≤ 0.05.
Exercise protocol
The training protocol consisted of a warm-up (10 minutes of jogging and light physical activity), the main exercises, and a cool-down (Five minutes of stretching). The circuit resistance training protocol was implemented three sessions a week, with each session involving 60 minutes of resistance bodyweight training (Table 2). The exercises were performed in three rounds, with 2-3 minutes of rest intervals between each round of running and the desired stations, at an intensity of 55-65% VO2max.
Results
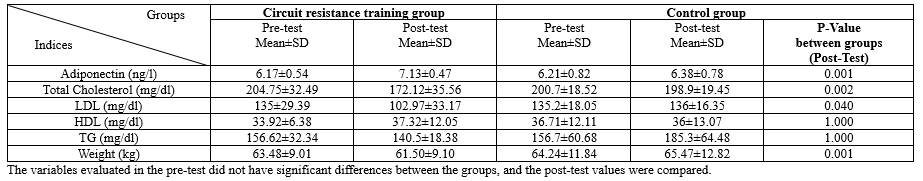
According to the results of the paired t-test, eight weeks of circuit resistance training significantly increased adiponectin levels (P-value=0.001) and significantly decreased LDL (P-value=0.031) and cholesterol (P-value =0.001). In addition, TG levels decreased, and HDL levels increased, although these changes were not considered significant (Table 2).
According to the obtained results, weight, fat percentage, and BMI significantly decreased in the training group, while no significant changes were observed in the control group. The results of the independent t-test also indicated significant differences between the study groups regarding changes in adiponectin levels, LDL, cholesterol, weight, fat percentage, and BMI (Table 2).
Obesity has increased significantly over the past few decades worldwide (1). Weight gain occurs due to an energy imbalance, with the body receiving more energy than needed (2). Adipose tissue dysfunction leads to conditions such as metabolic syndrome, cardiovascular disorders, and cancer in obese individuals (3). In general, metabolic syndrome is defined as the presence of at least three clinical risk factors, including abdominal (Visceral) obesity, hypertension, dyslipidemia, decreased high-density lipoproteins, high triglycerides (TGs), free fatty acids, low-density lipoproteins, and insulin resistance (4).
The adipose tissue was initially regarded as an energy storage organ; however, it has recently been identified as an active endocrine tissue (5). Adipose tissue produces and releases various pro-inflammatory and anti-inflammatory factors, including leptin, adiponectin, visfatin, resistin, and omentin, which function at autocrine, paracrine, and endocrine levels (6). Adiponectin is a protein composed of 244 amino acids and is found in the blood in three different molecular weights: low molecular weight, moderate molecular weight, and high molecular weight (7). High molecular weight is most significantly associated with obesity, cardiovascular diseases, and metabolic syndrome (8). Adiponectin activates adenosine monophosphate-activated protein kinase (AMPK) through the adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1. AMPK is primarily a signaling pathway that ultimately increases beta-oxidation, glucose transporter type 4, cluster of differentiation 36, and glucose and fatty acid intake by cells (8).
Adiponectin exerts its effects through two membrane receptors, AdipoR1 and AdipoR2; the former is primarily found in muscles, and the latter in the liver (9). In addition, adiponectin activates AMPK phosphorylation by binding to AdipoR1, thereby increasing glucose use and fatty acid oxidation in muscles and the liver (10). Adiponectin also activates PGC-1, a cell receptor that facilitates the release of mitochondrial proteins (11). PGC-1 regulates lipid metabolism and long-chain fatty acid oxidation through the expression of several carboxylic acid cycle genes and the mitochondrial fatty acid pathway (12). Furthermore, PGC-1 increases the expression of fibronectin type III domain-containing protein 5 (13). This receptor also stimulates mitochondrial biogenesis and angiogenesis while reducing atrophy.
Adiponectin has antidiabetic properties due to its ability to increase insulin sensitivity (14). Studies indicate decreased adiponectin levels in obese individuals, which lead to increased susceptibility to health conditions such as metabolic syndrome, insulin resistance, and type II diabetes (15). A negative correlation has also been reported between this protein and the body mass index (BMI) (16). Conversely, increased adiponectin levels can significantly reduce total cholesterol, low-density lipoprotein (LDL), and TGs while increasing high-density lipoprotein (HDL) (17).
Esposito et al. (2003) reported that aerobic exercise for two years in obese middle-aged women resulted in a decrease in body weight (BW) along with an increase in plasma adiponectin concentration. Contrary to these positive effects of exercise on adiponectin levels in the blood of obese individuals, Hara et al. (2005) suggested that complex exercise for eight weeks in obese adolescents did not induce changes in plasma adiponectin concentration, and Polak et al. (2006) reported that aerobic exercise for 12 weeks did not increase blood adiponectin concentration in obese individuals (18).
In a study, six weeks of acute aerobic training at an intensity of 40-50% VO2max was reported to have no significant effect on adiponectin (19). Another study assessed the effect of aerobic training with varied intensities on adiponectin, indicating that eight weeks of low-intensity aerobic exercise had no significant effect on adiponectin. Meanwhile, moderate- and high-intensity aerobic training were shown to significantly increase adiponectin. In a related study, one session of aerobic training at an intensity of 65% VO2max significantly reduced adiponectin (20).
In general, regular resistance training has been found to be effective in decreasing body fat in women and increasing adiponectin (21). High-intensity interval training significantly increases serum adiponectin (22). A study in this regard showed an increase in serum adiponectin levels following resistance and aerobic training, which improved inflammation and metabolic syndrome (23). Evidence suggests that regular physical activity also reduces TGs and LDL while increasing HDL (24). The most significant LDL reduction and HDL increase have been reported in individuals performing aerobic training at an intensity of 55-65% VO2max (24). Furthermore, resistance training has been shown to significantly affect the lipid profile. A study indicated a significant decrease in TG and LDL and a significant increase in HDL following resistance training exercises (25).
Recently, combined aerobic and resistance training has been suggested to have a more favorable impact on cardiovascular and general health compared to aerobic and resistance training performed separately (26). Circuit training, which is beneficial to overweight individuals, is a combination of aerobic and resistance training (27). These exercises increase muscle endurance and strength, as well as aerobic function (28). Previous studies have indicated that circuit training is associated with a significant decrease in blood pressure and an increase in muscle tissue, strength, and aerobic capacity (29). Moreover, circuit training has been reported to improve metabolism by increasing oxygen intake (29).
Considering these research findings, the effect of exercise type, intensity, and/or duration on blood adiponectin levels in obese individuals remains controversial. Therefore, the purpose of this study was to investigate the effect of eight weeks of resistance circuit training on plasma adiponectin levels and lipid profiles in overweight women.
Methods
This quasi-experimental research was performed with a pretest-posttest design and a control group. After the call, volunteers were invited to participate in the project through a public announcement. In addition, patients with medical records at the Research Institute were informed about and invited to the project through phone calls. The sample population included overweight women aged 25-45 years. Inclusion criteria were a BMI of 28-40 kg/m2, absence of specific diseases, and no regular sports activity within the past six months. Individuals with hereditary hypolipoproteinemia and other chronic diseases, such as diabetes mellitus, or those who were drug users, were excluded from the study. In total, 30 women with a mean age of 34±4 years, mean height of 165.35±7.5 centimeters, mean weight of 81.17±9.13 kilograms, and mean BMI of 30.17±2.13 kg/m2 were enrolled in the study. The participants were randomly divided into two groups: circuit resistance training (n=15) and control (n=15). The present research was conducted in Absard City in the winter of 2020.
In one session, the subjects were familiarized with the type of the study, its objectives, implementation methods, and possible risks, and informed consent was obtained. Data were collected using the Physical Activity Readiness Questionnaire prior to the research. Notably, 10 participants withdrew from the research for personal reasons. Finally, eight and 10 participants remained in the training and control groups, respectively. The study protocol was approved by the Ethics Committee of the Sports Sciences Research Institute (Code: IR.SSRC.REC.1398.114).
Anthropometric indices, including height, weight, fat percentage, and abdominal circumference, were measured. Height was measured using a tape measure with 0.5 cm precision, and the fat percentage of the participants was calculated using a skinfold caliper, which measures subcutaneous fat based on a three-site method (triceps, pelvis, and thigh) on the right side of the body, after applying the digits obtained by the Jackson and Pollock body density formula (30). Furthermore, maximum strength was measured using an indirect method involving leg and chest press movements to assess lower body and upper body strength, respectively (31).
Chemical variables were measured based on blood samples (5 cc) collected from the participants by a specialist at two stages: 48 hours before the first exercise session and 48 hours after the last exercise session. The samples were collected in a laboratory after 8-10 hours of fasting. After centrifuging the blood samples at 2,500 rpm/min, the samples were transferred to the Shahid Beheshti Endocrinology and Metabolism Research Institute in Tehran, Iran, in a special package at a specific temperature. The exercise protocol was performed by the training group in a multifunctional center, with three sessions per week for eight weeks. The control group was assigned no regular physical activity (Table 1).
With regard to the biochemical indices, adiponectin was measured using the ELISA assay with a sensitivity of 0.1 ng/l and an intragroup change coefficient of 7.2%, using the Zellbio GmbH kit (Made in Germany). TGs were measured using a colorimetric enzymatic method with a sensitivity of 1 mg/dl and an intragroup change coefficient of 2.3%, using the Pars test kit. Cholesterol was measured using an enzymatic photometric method with a sensitivity of 3 mg/dl and an intragroup change coefficient of 2.1%, using the Pars test kit. HDL was measured using an enzymatic photometric method with a sensitivity of 1 mg/dl and an intragroup change coefficient of 3.4%, using the Pars test kit. LDL was measured using the Friedewald formula (32).
Data analysis was performed in SPSS version 24 using the Shapiro-Wilk test to determine the normal distribution of data among the groups. In addition, Levene’s test was used to assess the homogeneity of variance between the groups. One-way analysis of variance (ANOVA) and dependent t-test were also applied at the significance level of P-value ≤ 0.05.
Exercise protocol
The training protocol consisted of a warm-up (10 minutes of jogging and light physical activity), the main exercises, and a cool-down (Five minutes of stretching). The circuit resistance training protocol was implemented three sessions a week, with each session involving 60 minutes of resistance bodyweight training (Table 2). The exercises were performed in three rounds, with 2-3 minutes of rest intervals between each round of running and the desired stations, at an intensity of 55-65% VO2max.
Results
According to the results of the paired t-test, eight weeks of circuit resistance training significantly increased adiponectin levels (P-value=0.001) and significantly decreased LDL (P-value=0.031) and cholesterol (P-value =0.001). In addition, TG levels decreased, and HDL levels increased, although these changes were not considered significant (Table 2).
According to the obtained results, weight, fat percentage, and BMI significantly decreased in the training group, while no significant changes were observed in the control group. The results of the independent t-test also indicated significant differences between the study groups regarding changes in adiponectin levels, LDL, cholesterol, weight, fat percentage, and BMI (Table 2).
Discussion
According to the results of the present study, eight weeks of circuit resistance training significantly decreased BW, BMI, fat percentage, LDL, and cholesterol, while significantly increasing adiponectin levels in the training group compared to the control group. However, no significant changes were observed in TG and HDL levels. Consistently, Ricci et al. reported increased adiponectin levels and improved insulin resistance after eight weeks of resistance training (33). According to Zamani et al., eight weeks of resistance training significantly increased adiponectin and testosterone levels in healthy male participants (34). Furthermore, Dehrashid et al. reported a significant increase in adiponectin levels after 12 weeks of circuit resistance training (35). Similarly, Dehrashid et al. observed a significant increase in adiponectin levels following 10 weeks of resistance training. In the mentioned research, the reduction in TG, LDL, and cholesterol was not considered significant (35).
According to the literature, long-term sports activities increase adiponectin levels (36). While all the studies in this regard involve eight weeks of training, the interventions range from high-intensity interval training to endurance training (36). Moreover, the duration of these interventions varies, with the number of weekly sessions ranging from a minimum of three per week (21,37) to a maximum of seven per week (38-40).
In a study conducted by Vardar et al., two training protocols commonly used in community training programs were compared. The proper exercise volume (Six sessions per week) was highlighted as a strength of the exercise protocol in the mentioned study, while the inadequate adiponectin measurement (Gene expression alone without assessing plasma adiponectin levels) was noted as a limitation (41). Studies conducted by Karajbani et al. (2018), Rostamizadeh et al. (2018), Hosseini et al. (2018), Mosaf and Abedi (2018), Ghaleno et al. (2017), Bettiol et al. (2017), Albashi et al. (2017), Piri and Zamani (2016), Nader et al. (2016), Bouri and Piri (2015), and Wang et al. (2015) were similar to the current research in terms of objectives and design. The common positive aspects of these studies included the number and type of participants (Obese and overweight individuals and patients) and the appropriate length of the interventions (8-20 weeks). However, the limitations of these studies included the use of the maximum heart rate index instead of maximum oxygen consumption in most cases, which might have reduced the accuracy of controlling training intensity and resulted in the relatively low volume of training (Three sessions per week).
Adiponectin is a protein hormone secreted by adipocytes that aids in insulin sensitivity and inflammation (18), regulating glucose metabolism in skeletal muscle and adipocytes through the activation of phosphorylated AMP-activated protein kinase and proliferator-activated receptors. Obese individuals have a lower plasma concentration of adiponectin compared to those with normal weight, and a reduction in BW and BFM increases adiponectin levels in the blood (18).
Controlling environmental confounding factors is one of the key strengths of the animal studies conducted by Vardar et al (2018) and Racil et al. (2013). In these studies, less attention was given to the type of diet, which could be considered a limitation. Meanwhile, high training intensity and the use of the maximum oxygen consumption index instead of the maximum heart rate increased the accuracy of these studies. The relatively small number of participants and training sessions might have also affected the results of the aforementioned studies (41,42).
Voss et al. (2016) conducted research on individuals with athletic bodies, reporting that exercise background could affect the adiponectin response to sports activities (43). Therefore, performing similar studies on non-athletic individuals could yield interesting results. In another study, Saunders et al. (2012) evaluated the effect of moderate-intensity running (Close to the anaerobic threshold) on obese individuals. One of the strengths of the mentioned study was its appropriate number of participants (n=38) and their condition (Obesity). Meanwhile, their exercise protocol (i.e., running) burns a considerable number of calories, which might have altered the adiponectin levels of the subjects more significantly. In addition, VO2max is considered a more accurate index for controlling exercise intensity (44).
Given the involvement of large muscles, multi-joint bodyweight resistance exercises increase energy consumption, which is essential for overweight individuals (45). Therefore, it seems that eight weeks of circuit training could increase adiponectin levels while saving time. Furthermore, these exercises could decrease LDL, cholesterol, body fat percentage, and weight by increasing energy consumption. It is suggested that multi-joint and aerobic exercises be incorporated into circuit training protocols for overweight individuals.
Conclusion
According to the findings of this study, an 8-week circuit resistance training program can be effective in improving adiponectin levels and lipid profile in obese women.
Acknowledgement
The authors of this article would like to express their gratitude to Semnan University and all the subjects who participated in this study.
Funding sources
Not applicable.
Ethical statement
The study protocol was approved by the Ethics Committee of the Sports Sciences Research Institute (Code: IR.SSRC.REC.1398.114).
Conflicts of interest
The authors have no conflicts of interest that are directly relevant to the content of this original research paper.
Author contributions
Sara Valikhani contributed to data collection, statistical analysis. Mehdi Hedayati contributed to laboratory experiments. Seyed Mohsen Avandi conceived and supervised the study. All authors read and approved the final manuscript.
Type of Article: Original article |
Subject:
Health
Received: 2023/08/8 | Accepted: 2024/06/10 | Published: 2025/09/2
Received: 2023/08/8 | Accepted: 2024/06/10 | Published: 2025/09/2
References
1. Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Paley CA, Johnson MI. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018;10(1):7. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Stanford KI, Goodyear LJ. Exercise regulation of adipose tissue. Adipocyte. 2016;5(2):153-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2(5):239-42. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Pierard M, Conotte S, Tassin A, Boutry S, Uzureau P, Boudjeltia KZ, et al. Interactions of exercise training and high-fat diet on adiponectin forms and muscle receptors in mice. Nutr Metab. 2016;13(1):75. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Ando D, Hosaka Y, Suzuki K, Yamagata Z. Effects of exercise training on circulating high molecular weight adiponectin and adiponectin oligomer composition: a randomized controlled trial. J Atheroscler Thromb. 2009;16(6):733-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Cheng KK, Lam KS, Wang B, Xu A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract Res Clin Endocrinol Metab. 2014;28(1):3-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99(25):16309-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464(7293):1313-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Cheng CF, Ku HC, Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19(11):3447. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Otvos Jr L. Potential adiponectin receptor response modifier therapeutics. Front Endocrinol (Lausanne). 2019;10:539. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13(1):103. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Kazemizadeh V, Behpour N. The effect of 30-hours sleep deprivation on the response of leptin and ghrelin levels to an exhaustive activity among active male students. Journal of Sabzevar University of Medical Sciences. 2021;28(4):569-80. [View at Publisher] [Google Scholar]
16. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015;18(5):430-42. [View at Publisher] [PMID] [Google Scholar]
18. Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor α in obese women. Metabolism. 2006;55(10):1375-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Bobbert T, Wegewitz U, Brechtel L, Freudenberg M, Mai K, Möhlig M, et al. Adiponectin oligomers in human serum during acute and chronic exercise: relation to lipid metabolism and insulin sensitivity. Int J Sports Med. 2007;28(1):1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Jamurtas AZ, Theocharis V, Koukoulis G, Stakias N, Fatouros I, Kouretas D, et al. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur J Appl Physiol. 2006;97(1):122-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Park KM, Park SC, Kang S. Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women. J Exerc Rehabil. 2019;15(5): 676-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Zarei F, Shadmehri S, Daryanoosh F, Sherafati Moghadam M, Mahmoodi MT. The effect of eight weeks of high-intensity interval training (HIIT) on the serum levels of chemerin, omentin-1 and apelin on overweight female Sprague-Dawley rats. SSU_Journals. 2018;26(6):473-82. [View at Publisher] [Google Scholar]
23. Sirico F, Bianco A, D'Alicandro G, Castaldo C, Montagnani S, Spera R, et al. Effects of physical exercise on adiponectin, leptin, and inflammatory markers in childhood obesity: systematic review and meta-analysis. Child Obes. 2018;14(4):207-17. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16(1):132. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48(1):9-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Getty AK, Wisdo TR, Chavis LN, Derella CC, McLaughlin KC, Perez AN, et al. Effects of circuit exercise training on vascular health and blood pressure. Prev Med Rep. 2018;10:106-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Al-Haliq M. Using the circuit training method to promote the physical fitness components of the hashemite university students. Advances in Physical Education. 2015;05(03):170-5. [View at Publisher] [DOI] [Google Scholar]
28. Klika B, Jordan C. High-intensity circuit training using body weight: Maximum results with minimal investment. ACSMs Health Fit J. 2013;17(3):8-13. [View at Publisher] [DOI] [Google Scholar]
29. Contrò V, Bianco A, Cooper J, Sacco A, Macchiarella A, Traina M, et al. Effects of different circuit training protocols on body mass, fat mass and blood parameters in overweight adults. Journal of Biological Research-Bollettino della Società Italiana di Biologia Sperimentale. 2017;90(1):10-2. [View at Publisher] [DOI] [Google Scholar]
30. Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. Journal of applied physiology. 2012;113(12):1831-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Liberman K, Forti LN, Beyer I, Bautmans I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: a systematic review. Curr Opin Clin Nutr Metab Care. 2017;20(1):30-53. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Karajibani M, Montazerifar F, Dehghani K, Mogharnasi M, Mousavi Gilan R, Dashipour A. The Effect of Endurance Exercise Training on Vaspin, Lipid Profile, and Anthropometric Indices in Young People. Journal of Nutrition and Food Security. 2019;4(4):263-71. [View at Publisher] [DOI] [Google Scholar]
33. Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: a comparative review. Vet J. 2012;191(3):292-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Zamani M, Peeri M, Azarbayjani MA, Matinhomaee H. Effects of Resistance Training on Adiponectin, Testosterone and Cortisol Levels in Untrained Men. Med Lab J. 2016;10(4):24-31. [View at Publisher] [DOI] [Google Scholar]
35. Dehrashid KA, Siahkohian M, Ahmadi S, Bolboli L. Comparison and study of the effects of resistance exercise trainings with two different loadings with Omega3-6-9 supplement on Adiponectin and hsCRP in Healthy overweight young men. 2018;23(5):21-31. [View at Publisher] [DOI] [Google Scholar]
36. Simpson KA, Singh MAF. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring). 2008;16(2):241-56. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Shalamzari SA, Daneshfar A, Sablouei MH, Fiatarone MA. The Effect of Aerobic Training on Tumor Growth, Adiponectin, Leptin and Ghrelin in Mice with Breast Cancer. Iranian Red Crescent Medical Journal. 2018;20(2):1-10. [View at Publisher] [DOI] [Google Scholar]
38. Ahmadizad S, Ghorbani S, Ghasemikaram M, Bahmanzadeh M. Effects of short-term nonperiodized, linear periodized and daily undulating periodized resistance training on plasma adiponectin, leptin and insulin resistance. Clin Biochem. 2014;47(6):417-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Baitul M, Susanto H, Kushartanti W, Rahayu S, editors. Beneficial Health Effect of Aquarobics (Role of Adiponectin on Women with Obesity). IOP Conf Series: Materials Science and Engineering; 1st Annual Applied Science and Engineering Conference. 2017;180. [View at Publisher] [DOI] [Google Scholar]
40. Shahriari M, Abbasi S, Moghzi P, Moeini M. Relation Between Health Promotion Behaviors Lifestyle and Islamic Life-style in Patients With Acute Coronary Syndrome. Journal of Clinical Nursing and Midwifery. 2018;7(2):126-37. [View at Publisher] [Google Scholar]
41. Vardar SA, Karaca A, Güldiken S, Palabıyık O, Süt N, Demir AM. High-intensity interval training acutely alters plasma adipokine levels in young overweight/obese women. Arch Physiol Biochem. 2018;124(2):149-55. [View at Publisher] [DOI] [PMID] [Google Scholar]
42. Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
43. Voss S, Nikolovski Z, Bourdon P, Alsayrafi M, Schumacher Y. The effect of cumulative endurance exercise on leptin and adiponectin and their role as markers to monitor training load. Biol Sport. 2016;33(1):23-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
44. Saunders TJ, Palombella A, McGuire KA, Janiszewski PM, Després JP, Ross R. Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab. 2012;2012:148729. [View at Publisher] [DOI] [PMID] [Google Scholar]
45. Paoli A. Resistance training: the multifaceted side of exercise. Am J Physiol Endocrinol Metab. 2012;302(3):E387. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |