Volume 11, Issue 1 (7-2023)
Jorjani Biomed J 2023, 11(1): 16-19 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirzaei Ashrafi F, Avandi S M, Khaleghian A. Comparison of twelve weeks periodized circuit resistance training and traditional resistance training on calprotectin levels in obese men. Jorjani Biomed J 2023; 11 (1) :16-19
URL: http://goums.ac.ir/jorjanijournal/article-1-946-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-946-en.html
1- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,m.avandi@semnan.ac.ir
3- Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,
3- Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences, Iran
Full-Text [PDF 526 kb]
(2317 Downloads)
| Abstract (HTML) (6583 Views)
Full-Text: (1306 Views)
Introduction
Obesity is a significant risk factor for hypertension, type II diabetes, hyperlipidemia, insulin resistance, and coronary artery disease (1). Obesity could be identified by chronic inflammation (2). Chronic inflammation constantly generates an initial pro-inflammatory immune response by developing the adipose tissue during Long-term obesity (3). Researchers believe that metabolic inflammation caused by macrophages plays a key role in insulin resistance. Adipose tissue macrophages could be converted from an anti-inflammatory range to widespread inflammatory factors. Anti-inflammatory cytokines, including interleukin 10 (IL-10) and calprotectin, regulate inflammatory and immune responses (4). A significant amount of calprotectin is released with the activation of neutrophils and the adhesion of monocytes to the endothelium (5, 6).
Studies have shown a significant increase in the plasma levels of calprotectin in some inflammatory diseases, which causes the protein to be considered a suitable marker for inflammatory processes (7). Obesity and type II diabetes are often characterized by increased calprotectin concentrations in the plasma and urine (8). A sedentary lifestyle and obesity also lead to mild chronic inflammation and increased levels of inflammatory markers in plasma (9).
Lifestyle modification and increasing nutritional knowledge among women using appropriate methods reduce the prevalence of obesity (10). Physical activity seems to prevent the rise of inflammatory markers appropriately. In this regard, resistance training has been recommended to establish physiological adaptations and prevent the rise of inflammatory markers (11). long term traditional resistance training could affect the inflammatory factors involved in the metabolic syndrome (12). On the other hand, circuit resistance training is designed to have the characteristics of resistance training and challenge the aerobic energy system. As a result, they affect different body functions, stimulate various enzymes, and change the serum levels of these enzymes (13, 14). Moreover, physical activity (e.g., resistance training) may induce significant hormonal, physiological, immunological, and metabolic changes in the body, affecting the immune system markers (15).
While the mechanism of the effectiveness of resistance training is not generally clear, it could be stated that resistance training alters the production of inflammatory and anti-inflammatory cytokines and improves insulin resistance, depending on the intensity of the training. Accordingly, there are solid logical reasons for attention to resistance training to prevent or treat obesity (16). Olsen et al. (2007) showed that resistance training could reduce the inflammatory indices in overweight women (17). Furthermore, in a study by Aile et al. (2017), resistance training improved the enzymatic markers of the liver in obese men (18). In another research, Fagerhol et al. (2005) observed a significant increase in the plasma levels of neutrophil-derived calprotectin after intense physical exercises. In addition, they reported a significant correlation between the increase in calprotectin levels and the duration of competitions (19). Mehrabi et al. (2016) also showed that circuit resistance training decreases inflammatory indices, causing metabolic and immune function disorders after the intervention (20).
The present study aimed to determine the effects of two types of resistance training on the serum calprotectin levels of obese men. Does circuit resistance training protocol have different effects on inflammatory indices in obese men compared to traditional resistance protocol?
Methods
Participants
This applied, quasi-experimental study was conducted with a pre-test-post-test design. The subjects included sedentary young men (17-23 years) living in Semnan, Iran, who had no regular physical activities within the previous six months. After an announcement, voluntary participants completed their disease history, demographic characteristics, and level of physical activity questionnaire. Informed consent was also obtained from the participants.
Thirty-three subjects were selected via random sampling and divided into three groups (n=11), including two experimental groups and a control group. The inclusion criteria of the study were as follows: 1) age of 17-23 years; 2) no regular physical activities within the past six months; 3) no developmental and motor disorders; 4) no cardiovascular diseases and 5) male gender. The exclusion criteria were unwillingness to participate and absence in more than one session in the training protocols. The present study was approved and registered by the Ethics Committee of Mazandaran University (IR.UMZ.REC.1399.017) and the Iranian Clinical Trial Center (IRCT) with the code IRCT20180928041160N2.
The nutritional status of the subjects was monitored before the pre-test and post-test, which may affect some of the measured factors. Pre-test measurements included anthropometric parameters (height, weight, fat thickness, BMI, body fat percentage, and lean body mass) and one-repetition maximum (1RM) determined in the selected exercise a week before the main protocols. Notably, 1RM was determined to monitor the intensity of the training and familiarize the subjects with exercises. The training protocol was performed based on the new 1RM for the second and third stages to apply the overload principle.
Training protocols
The Training protocol was a 12-week resistance training program (Circuit and traditional resistance training) in three weekly sessions, which continued in three stages (four-week). Training intensity was 50-80% 1RM, three sets/circular, 8-24 repetitions, and 8-14 exercises, respectively (Leg press, bench press, step up, incline bench press, Lat pull down, crunch, lunges, shoulder press, leg curl, seated row, leg extensions, reverse fly, knee hip raise on parallel, and barbell curl) with a wave pattern. The intensity variable that determined the quality of each training session was selected based on various references to choose the most appropriate training intensity for high metabolic pressure and affecting metabolism. The exercises had to be performed with proper intensity for maximum effectiveness (21, 22) (Figure 1).

The training volume in the two protocols was changed in the same way in different stages by changing the number of exercises performed in the sessions and different exercise orders.
Accordingly, the selected exercises ordered large-to-small muscles. On the other hand, the exercises were selected from multi-joint and single-joint exercises for each group. The multi-joint exercises were initially performed, and the single-joint exercises were performed afterward.
Before the second special stage, a new 1RM was determined for the second stage to apply the overload principle. In the first four weeks of the special exercises, three sets of 10-12 exercises were performed at 60-70% 1RM intensity with 15-20 repetitions for three weekly sessions with a wave pattern. The exercise volume and intensity increased in the seventh week by adding exercises.
In the Traditional exercises, the rest interval between the sets and exercises was 90 seconds and three minutes, respectively. In the circuit training, the rest interval between each circuit was three minutes during the entire training period. Before the third stage, a new 1RM was performed to apply the overload principle. The training protocol continued in three sessions per week with a wave pattern. In addition, 12-14 exercises were performed at the intensity of 70-80% 1RM in three sets with 8-15 repetitions in the form of two resistance protocols. In the seventh week, a shock was introduced to the intensity and volume of the exercises so that the physiological responses to the exercise episodes would not be flat. This arrangement was equal for the traditional and circuit resistance training protocols, and the only differences were the rest interval between the sets, the exercises in the traditional protocol, and the rest interval between each circuit in the circuit resistance protocol.
Blood samples were obtained in a fasting condition. Six milliliters of blood were collected from the anticoagulant vein. The blood samples were centrifuged at 3,000 rpm/min (1,000 g) at room temperature for 15 minutes. The serum samples were preserved at -80°C until sent to the laboratory. At the end of the training, blood samples were obtained from the participants again. Calprotectin levels were measured using an ELISA kit following the instructions of the manufacturer (COD number).
Data analysis was performed using descriptive statistics (mean and standard deviation) and Analytical statistics, including the Kolmogorov-Smirnov test to assess the normal distribution of the data, Levene's test to determine the equality of the variances, analysis of variance (ANOVA), One-way ANOVA, and paired sample t-test.
Results
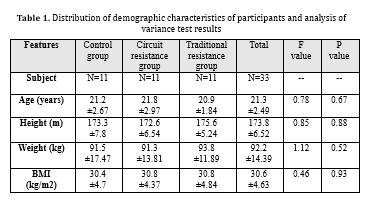
In the current study, the twelve-week circuit and traditional resistance training significantly increased calprotectin serum levels in young obese men (P=0.001). Furthermore, the results indicated a significant difference in calprotectin serum levels between the circuit and traditional resistance training groups (P=0.01). (Table 1) shows the mean demographic variables of the participants in three groups. According to (Table 1), the three study groups had no significant differences in demographic characteristics.
The composite ANOVA of the three (groups) × two (test stages) was used to evaluate the effects of the training protocols on calprotectin levels. According to the results, the test stages had a significant effect (F1.0, 30.0=121.29; P=0.00001; η2=0.80). Moreover, the effect of the test stages was significant in association with the group factor (F2.0, 30.0=69.86; P=0.00001; η2=0.82). However, the main effects were disregarded due to the significance of the interactional effects (test stages × group). One-way ANOVA was used to evaluate the performance of the two groups in the pre-test and post-test stages.
No significant difference was observed between the circuit and traditional training groups and the control group at the pre-test stage (P=0.08). However, a significant difference was seen between the three groups at the post-test stage (P=0.001). The Bonferroni test was used to determine the difference in the performance of the three groups, and the results indicated a significant difference
between the circuit and traditional resistance training groups in this regard (P=0.01). Considering the difference of means (4.05), the performance of the circuit resistance training group was greater than the traditional training group. Furthermore, a significant difference was observed between the circuit resistance training and control groups (P=0.001). Based on the difference of means (12.64), the performance of the circuit resistance training group was superior to the control group. A significant difference in performance was also denoted between the traditional training and control groups (P=0.02). Considering the difference of means (8.68), the performance of the traditional training group was superior to the control group. At this stage, paired sample t-test was used to evaluate the intragroup effects in each group separately (Table 2).
According to (Table 2), the dependent t-test results indicated progress in the circuit (P=0.0001) and traditional resistance training groups (P=0.001) regarding calprotectin levels at the pre-test stage (Figure 2). However, no significant difference in calprotectin levels was observed in the control group.

Discussion
The present study aimed to evaluate the effect of 12 weeks of circuit and traditional resistance training on calprotectin levels in obese men. According to the results, the 12-week circuit and traditional resistance training programs significantly affected the calprotectin levels. Moreover, the performance of the circuit resistance training group was superior to the traditional resistance training group.
In the current research, calprotectin plasma levels significantly increased in young obese men in response to the intervention. A literature review revealed that no other research has used these protocols. Therefore, limited evidence is available regarding the effects of long-term circuit resistance training and traditional resistance training on calprotectin levels. In another research, Fagerhol et al. reported a significant increase in the calprotectin levels derived from neutrophils after long-distance running (19). Similarly, Azarmehr et al. evaluated the effects of circuit resistance training on the inflammatory responses of obese men (23). In the mentioned study, 17 obese men (BMI>30 kg/m2) were selected and divided into two groups. The circuit resistance training program included eight stations with increasing intensity (65% to 85% 1RM). Body composition, the plasma levels of chemerin, and C-reactive protein (CRP) were measured twenty-four hours before and forty-eight hours after the protocol. According to the results, circuit resistance training improved the participants' body composition, thereby decreasing the plasma levels of chemerin and CRP (23).
Takahashi et al. assessed calprotectin concentrations at different sports levels and reported no significant changes in the calprotectin concentrations in most sports levels (24). This finding could be attributed to the shorter duration of training in the mentioned study. According to these scholars, exercise duration had the most significant effect on the changes in the active markers of leukocytes compared to training intensity (24).
Inflammatory processes originate from the adipose tissue, are abundantly found within the adipose tissue, and cause the production of various Cytokines. Calprotectin is one of the cytokines that has recently attracted the attention of researchers. Exercise is believed to reduce the levels of some pro-inflammatory and inflammatory cytokines, such as IL-6, TNF-α, and CRP (4). In the adipose tissue of obese individuals, pro-inflammatory cytokines (e.g., calprotectin) could cause extensive proliferation in the adipose tissue via paracrine signaling, thereby impairing the regulation of adipocytokines (25).
High calprotectin levels correlate directly with BMI, visceral fat, white blood cell count, and subcutaneous fat (26). Calprotectin is known to be actively secreted from the phagocytes into the bloodstream in response to an external stress stimulus (e.g., training), and high serum levels of this protein are associated with inflammatory disorders such as acute coronary syndrome and autoimmune diseases (27). Since neutrophil cells are the primary source of plasma calprotectin, and some researchers have reported increased leukocytes after intense physical activity, prolonged exercise is expected to increase calprotectin levels as a pro-inflammatory cytokine (28).
In order to justify the mechanism of the effects of resistance training on calprotectin levels in obese individuals, the effects of exercise on modulating fat stores, insulin activity, and general metabolism of the body should be mentioned; resistance training may have a positive effect on these parameters. Furthermore, exercise may play a pivotal role in reducing the concentration of pro-inflammatory agents and inflammation after exercise by improving glucose metabolism and insulin resistance and stimulating glucose uptake into the muscles.
In line with our findings, Fico et al. reported that acute exercise might reduce the transient inflammatory responses in the plasma as opposed to moderate exercise (25). In addition, plasma calprotectin increases with moderate-intensity exercise, indicating an inflammatory response. In another study, Vosugh evaluated the effects of eight weeks of aerobic training on the plasma calprotectin levels of 13 overweight postmenopausal women (29). The intervention consisted of twenty-four aerobic training sessions, which were performed in eight weeks at the intensity of 40-80% HRmax, and the duration of each session was 25-45 minutes. According to the study results, training increased calprotectin levels in overweight postmenopausal women. Furthermore, no significant correlations were observed between the insulin content, insulin sensitivity index, and calprotectin plasma levels (29).
The main limitation of the present study was the small sample size. Therefore, it is recommended that further investigations be conducted on larger sample sizes.
According to the results of the present study, a 12-week intervention course of the circuit and traditional resistance training affected the calprotectin levels of obese men. However, circuit resistance training had more significant effects than traditional resistance training.
Conclusion
According to the findings of this study, a 12-week periodized circuit resistance training and traditional resistance training can be effective on calprotectin levels in obese men.
Acknowledgement
The authors of this article would like to express their gratitude to Semnan University of Medical Sciences and all the subjects who participated in this study.
Funding sources
Financial support was not used to conduct the present research.
Ethical statement
The present study was approved and registered by the Ethics Committee of Mazandaran University (IR.UMZ.REC.1399.017) and the Iranian Clinical Trial Center (IRCT) with the code IRCT20180928041160N2.
Conflicts of interest
The authors have no conflict of interest that are directly relevant to the content of this original research paper.
Author contributions
The authors of this article have contributed to study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis and administrative, technical, and material support.
Obesity is a significant risk factor for hypertension, type II diabetes, hyperlipidemia, insulin resistance, and coronary artery disease (1). Obesity could be identified by chronic inflammation (2). Chronic inflammation constantly generates an initial pro-inflammatory immune response by developing the adipose tissue during Long-term obesity (3). Researchers believe that metabolic inflammation caused by macrophages plays a key role in insulin resistance. Adipose tissue macrophages could be converted from an anti-inflammatory range to widespread inflammatory factors. Anti-inflammatory cytokines, including interleukin 10 (IL-10) and calprotectin, regulate inflammatory and immune responses (4). A significant amount of calprotectin is released with the activation of neutrophils and the adhesion of monocytes to the endothelium (5, 6).
Studies have shown a significant increase in the plasma levels of calprotectin in some inflammatory diseases, which causes the protein to be considered a suitable marker for inflammatory processes (7). Obesity and type II diabetes are often characterized by increased calprotectin concentrations in the plasma and urine (8). A sedentary lifestyle and obesity also lead to mild chronic inflammation and increased levels of inflammatory markers in plasma (9).
Lifestyle modification and increasing nutritional knowledge among women using appropriate methods reduce the prevalence of obesity (10). Physical activity seems to prevent the rise of inflammatory markers appropriately. In this regard, resistance training has been recommended to establish physiological adaptations and prevent the rise of inflammatory markers (11). long term traditional resistance training could affect the inflammatory factors involved in the metabolic syndrome (12). On the other hand, circuit resistance training is designed to have the characteristics of resistance training and challenge the aerobic energy system. As a result, they affect different body functions, stimulate various enzymes, and change the serum levels of these enzymes (13, 14). Moreover, physical activity (e.g., resistance training) may induce significant hormonal, physiological, immunological, and metabolic changes in the body, affecting the immune system markers (15).
While the mechanism of the effectiveness of resistance training is not generally clear, it could be stated that resistance training alters the production of inflammatory and anti-inflammatory cytokines and improves insulin resistance, depending on the intensity of the training. Accordingly, there are solid logical reasons for attention to resistance training to prevent or treat obesity (16). Olsen et al. (2007) showed that resistance training could reduce the inflammatory indices in overweight women (17). Furthermore, in a study by Aile et al. (2017), resistance training improved the enzymatic markers of the liver in obese men (18). In another research, Fagerhol et al. (2005) observed a significant increase in the plasma levels of neutrophil-derived calprotectin after intense physical exercises. In addition, they reported a significant correlation between the increase in calprotectin levels and the duration of competitions (19). Mehrabi et al. (2016) also showed that circuit resistance training decreases inflammatory indices, causing metabolic and immune function disorders after the intervention (20).
The present study aimed to determine the effects of two types of resistance training on the serum calprotectin levels of obese men. Does circuit resistance training protocol have different effects on inflammatory indices in obese men compared to traditional resistance protocol?
Methods
Participants
This applied, quasi-experimental study was conducted with a pre-test-post-test design. The subjects included sedentary young men (17-23 years) living in Semnan, Iran, who had no regular physical activities within the previous six months. After an announcement, voluntary participants completed their disease history, demographic characteristics, and level of physical activity questionnaire. Informed consent was also obtained from the participants.
Thirty-three subjects were selected via random sampling and divided into three groups (n=11), including two experimental groups and a control group. The inclusion criteria of the study were as follows: 1) age of 17-23 years; 2) no regular physical activities within the past six months; 3) no developmental and motor disorders; 4) no cardiovascular diseases and 5) male gender. The exclusion criteria were unwillingness to participate and absence in more than one session in the training protocols. The present study was approved and registered by the Ethics Committee of Mazandaran University (IR.UMZ.REC.1399.017) and the Iranian Clinical Trial Center (IRCT) with the code IRCT20180928041160N2.
The nutritional status of the subjects was monitored before the pre-test and post-test, which may affect some of the measured factors. Pre-test measurements included anthropometric parameters (height, weight, fat thickness, BMI, body fat percentage, and lean body mass) and one-repetition maximum (1RM) determined in the selected exercise a week before the main protocols. Notably, 1RM was determined to monitor the intensity of the training and familiarize the subjects with exercises. The training protocol was performed based on the new 1RM for the second and third stages to apply the overload principle.
Training protocols
The Training protocol was a 12-week resistance training program (Circuit and traditional resistance training) in three weekly sessions, which continued in three stages (four-week). Training intensity was 50-80% 1RM, three sets/circular, 8-24 repetitions, and 8-14 exercises, respectively (Leg press, bench press, step up, incline bench press, Lat pull down, crunch, lunges, shoulder press, leg curl, seated row, leg extensions, reverse fly, knee hip raise on parallel, and barbell curl) with a wave pattern. The intensity variable that determined the quality of each training session was selected based on various references to choose the most appropriate training intensity for high metabolic pressure and affecting metabolism. The exercises had to be performed with proper intensity for maximum effectiveness (21, 22) (Figure 1).

The training volume in the two protocols was changed in the same way in different stages by changing the number of exercises performed in the sessions and different exercise orders.
Accordingly, the selected exercises ordered large-to-small muscles. On the other hand, the exercises were selected from multi-joint and single-joint exercises for each group. The multi-joint exercises were initially performed, and the single-joint exercises were performed afterward.
Before the second special stage, a new 1RM was determined for the second stage to apply the overload principle. In the first four weeks of the special exercises, three sets of 10-12 exercises were performed at 60-70% 1RM intensity with 15-20 repetitions for three weekly sessions with a wave pattern. The exercise volume and intensity increased in the seventh week by adding exercises.
In the Traditional exercises, the rest interval between the sets and exercises was 90 seconds and three minutes, respectively. In the circuit training, the rest interval between each circuit was three minutes during the entire training period. Before the third stage, a new 1RM was performed to apply the overload principle. The training protocol continued in three sessions per week with a wave pattern. In addition, 12-14 exercises were performed at the intensity of 70-80% 1RM in three sets with 8-15 repetitions in the form of two resistance protocols. In the seventh week, a shock was introduced to the intensity and volume of the exercises so that the physiological responses to the exercise episodes would not be flat. This arrangement was equal for the traditional and circuit resistance training protocols, and the only differences were the rest interval between the sets, the exercises in the traditional protocol, and the rest interval between each circuit in the circuit resistance protocol.
Blood samples were obtained in a fasting condition. Six milliliters of blood were collected from the anticoagulant vein. The blood samples were centrifuged at 3,000 rpm/min (1,000 g) at room temperature for 15 minutes. The serum samples were preserved at -80°C until sent to the laboratory. At the end of the training, blood samples were obtained from the participants again. Calprotectin levels were measured using an ELISA kit following the instructions of the manufacturer (COD number).
Data analysis was performed using descriptive statistics (mean and standard deviation) and Analytical statistics, including the Kolmogorov-Smirnov test to assess the normal distribution of the data, Levene's test to determine the equality of the variances, analysis of variance (ANOVA), One-way ANOVA, and paired sample t-test.
Results
In the current study, the twelve-week circuit and traditional resistance training significantly increased calprotectin serum levels in young obese men (P=0.001). Furthermore, the results indicated a significant difference in calprotectin serum levels between the circuit and traditional resistance training groups (P=0.01). (Table 1) shows the mean demographic variables of the participants in three groups. According to (Table 1), the three study groups had no significant differences in demographic characteristics.
The composite ANOVA of the three (groups) × two (test stages) was used to evaluate the effects of the training protocols on calprotectin levels. According to the results, the test stages had a significant effect (F1.0, 30.0=121.29; P=0.00001; η2=0.80). Moreover, the effect of the test stages was significant in association with the group factor (F2.0, 30.0=69.86; P=0.00001; η2=0.82). However, the main effects were disregarded due to the significance of the interactional effects (test stages × group). One-way ANOVA was used to evaluate the performance of the two groups in the pre-test and post-test stages.

No significant difference was observed between the circuit and traditional training groups and the control group at the pre-test stage (P=0.08). However, a significant difference was seen between the three groups at the post-test stage (P=0.001). The Bonferroni test was used to determine the difference in the performance of the three groups, and the results indicated a significant difference
between the circuit and traditional resistance training groups in this regard (P=0.01). Considering the difference of means (4.05), the performance of the circuit resistance training group was greater than the traditional training group. Furthermore, a significant difference was observed between the circuit resistance training and control groups (P=0.001). Based on the difference of means (12.64), the performance of the circuit resistance training group was superior to the control group. A significant difference in performance was also denoted between the traditional training and control groups (P=0.02). Considering the difference of means (8.68), the performance of the traditional training group was superior to the control group. At this stage, paired sample t-test was used to evaluate the intragroup effects in each group separately (Table 2).
According to (Table 2), the dependent t-test results indicated progress in the circuit (P=0.0001) and traditional resistance training groups (P=0.001) regarding calprotectin levels at the pre-test stage (Figure 2). However, no significant difference in calprotectin levels was observed in the control group.

Discussion
The present study aimed to evaluate the effect of 12 weeks of circuit and traditional resistance training on calprotectin levels in obese men. According to the results, the 12-week circuit and traditional resistance training programs significantly affected the calprotectin levels. Moreover, the performance of the circuit resistance training group was superior to the traditional resistance training group.
In the current research, calprotectin plasma levels significantly increased in young obese men in response to the intervention. A literature review revealed that no other research has used these protocols. Therefore, limited evidence is available regarding the effects of long-term circuit resistance training and traditional resistance training on calprotectin levels. In another research, Fagerhol et al. reported a significant increase in the calprotectin levels derived from neutrophils after long-distance running (19). Similarly, Azarmehr et al. evaluated the effects of circuit resistance training on the inflammatory responses of obese men (23). In the mentioned study, 17 obese men (BMI>30 kg/m2) were selected and divided into two groups. The circuit resistance training program included eight stations with increasing intensity (65% to 85% 1RM). Body composition, the plasma levels of chemerin, and C-reactive protein (CRP) were measured twenty-four hours before and forty-eight hours after the protocol. According to the results, circuit resistance training improved the participants' body composition, thereby decreasing the plasma levels of chemerin and CRP (23).
Takahashi et al. assessed calprotectin concentrations at different sports levels and reported no significant changes in the calprotectin concentrations in most sports levels (24). This finding could be attributed to the shorter duration of training in the mentioned study. According to these scholars, exercise duration had the most significant effect on the changes in the active markers of leukocytes compared to training intensity (24).
Inflammatory processes originate from the adipose tissue, are abundantly found within the adipose tissue, and cause the production of various Cytokines. Calprotectin is one of the cytokines that has recently attracted the attention of researchers. Exercise is believed to reduce the levels of some pro-inflammatory and inflammatory cytokines, such as IL-6, TNF-α, and CRP (4). In the adipose tissue of obese individuals, pro-inflammatory cytokines (e.g., calprotectin) could cause extensive proliferation in the adipose tissue via paracrine signaling, thereby impairing the regulation of adipocytokines (25).
High calprotectin levels correlate directly with BMI, visceral fat, white blood cell count, and subcutaneous fat (26). Calprotectin is known to be actively secreted from the phagocytes into the bloodstream in response to an external stress stimulus (e.g., training), and high serum levels of this protein are associated with inflammatory disorders such as acute coronary syndrome and autoimmune diseases (27). Since neutrophil cells are the primary source of plasma calprotectin, and some researchers have reported increased leukocytes after intense physical activity, prolonged exercise is expected to increase calprotectin levels as a pro-inflammatory cytokine (28).
In order to justify the mechanism of the effects of resistance training on calprotectin levels in obese individuals, the effects of exercise on modulating fat stores, insulin activity, and general metabolism of the body should be mentioned; resistance training may have a positive effect on these parameters. Furthermore, exercise may play a pivotal role in reducing the concentration of pro-inflammatory agents and inflammation after exercise by improving glucose metabolism and insulin resistance and stimulating glucose uptake into the muscles.
In line with our findings, Fico et al. reported that acute exercise might reduce the transient inflammatory responses in the plasma as opposed to moderate exercise (25). In addition, plasma calprotectin increases with moderate-intensity exercise, indicating an inflammatory response. In another study, Vosugh evaluated the effects of eight weeks of aerobic training on the plasma calprotectin levels of 13 overweight postmenopausal women (29). The intervention consisted of twenty-four aerobic training sessions, which were performed in eight weeks at the intensity of 40-80% HRmax, and the duration of each session was 25-45 minutes. According to the study results, training increased calprotectin levels in overweight postmenopausal women. Furthermore, no significant correlations were observed between the insulin content, insulin sensitivity index, and calprotectin plasma levels (29).
The main limitation of the present study was the small sample size. Therefore, it is recommended that further investigations be conducted on larger sample sizes.
According to the results of the present study, a 12-week intervention course of the circuit and traditional resistance training affected the calprotectin levels of obese men. However, circuit resistance training had more significant effects than traditional resistance training.
Conclusion
According to the findings of this study, a 12-week periodized circuit resistance training and traditional resistance training can be effective on calprotectin levels in obese men.
Acknowledgement
The authors of this article would like to express their gratitude to Semnan University of Medical Sciences and all the subjects who participated in this study.
Funding sources
Financial support was not used to conduct the present research.
Ethical statement
The present study was approved and registered by the Ethics Committee of Mazandaran University (IR.UMZ.REC.1399.017) and the Iranian Clinical Trial Center (IRCT) with the code IRCT20180928041160N2.
Conflicts of interest
The authors have no conflict of interest that are directly relevant to the content of this original research paper.
Author contributions
The authors of this article have contributed to study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis and administrative, technical, and material support.
Type of Article: Original article |
Subject:
Health
Received: 2022/12/30 | Accepted: 2023/07/16 | Published: 2023/07/1
Received: 2022/12/30 | Accepted: 2023/07/16 | Published: 2023/07/1
References
1. Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary care interventions for obesity: review of the evidence. Curr Obes Rep. 2019;8:128-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Rojas K, Matthews N, Raker C, Clark MA, Onstad M, Stuckey A, et al. Body mass index (BMI), postoperative appearance satisfaction, and sexual function in breast cancer survivorship. J Cancer Surviv. 2018;12:127-33. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. The Journal of clinical investigation. 2017;127(1):1-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36-48. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, LeBoeuf RC, et al. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123(11):1216-26. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Tøn H, Brandsnes Ø, Dale S, Holtlund J, Skuibina E, Schjønsby H, et al. Improved assay for fecal calprotectin. Clinica chimica acta. 2000;292(1-2):41-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218-24. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Pedersen L, Nybo M, Poulsen MK, Henriksen JE, Dahl J, Rasmussen LM. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14(1):196. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Xu H. Obesity and metabolic inflammation. Drug Discov Today Dis Mech. 2013;10(1-2):e21-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Pasdar Y, Darbandi M, Niazi P, Alghasi S, Roshanpour F. The Prevalence and the Affecting Factors of Obesity in Women of Kermanshah. Jorjani Biomed J. 2015;3(1):82-97. [View at Publisher] [Google Scholar]
11. Strasser B, Arvandi M, Siebert U. Resistance training, visceral obesity and inflammatory response: a review of the evidence. Obes Rev. 2012;13(7):578-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Nicholas L, Morrison J, Rattanatray L, Zhang S, Ozanne S, McMillen I. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond). 2016;40(2):229-38. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Flack K, Davy K, Hulver M, Frisard M, Anderson A, Boutagy N, et al. Resistance Training and Mitochondrial Metabolism. The FASEB Journal. 2015;29(s1):LB363. [View at Publisher] [DOI] [Google Scholar]
14. 14.Fleck SJ, Kraemer W. Designing resistance training programs. 4th ed. US: Human Kinetics; 2014. [View at Publisher] [Google Scholar]
15. Knowles OE, Aisbett B, Main LC, Drinkwater EJ, Orellana L, Lamon S. Resistance training and skeletal muscle protein metabolism in eumenorrheic females: implications for researchers and practitioners. Sports Medicine. 2019;49(11):1637-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Tresierras MA, Balady GJ. Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev. 2009;29(2):67-75. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Olson TP, Dengel D, Leon A, Schmitz K. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond). 2007;31(6):996-1003. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Alie M, Matinhomaee H, Peeri M. The effect of different resistance training intensities on liver function in obese men. Journal of Sport Biosciences. 2017;9(1):75-92. [View at Publisher] [Google Scholar]
19. Fagerhol M, Nielsen H, Vetlesen A, Sandvik K, Lyberg T. Increase in plasma calprotectin during long‐distance running. Scand J Clin Lab Invest. 2005;65(3):211-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Mehrabani J, Mirmohamadloo F, Nobari H. The Effect of 8 Weeks of Circuit Resistance Training on Ox-LDL, hs-CRP, HbA1c and Insulin Resistance Index in Sedentary Postmenopausal Women. Sport Physiology & Management Investigations. 2016;8(4):47-57. [View at Publisher] [Google Scholar]
21. Maté-Muñoz JL, Monroy AJA, Jiménez PJ, Garnacho-Castaño MV. Effects of instability versus traditional resistance training on strength, power and velocity in untrained men. J Sports Sci Med. 2014;13(3):460-8. [View at Publisher] [Google Scholar]
22. Moradi F. Changes of serum adiponectin and testosterone concentrations following twelve weeks resistance training in obese young men. Asian J Sports Med. 2015;6(4):e23808. [View at Publisher] [DOI] [Google Scholar]
23. Azarmehr SA, Toloee MR, Akbari E. The effect of 8 weeks of Circuit Resistance Training on metabolic syndrome risk factors and body composition in women over age 50 with diabetes mellitus type 2. International Journal of Applied Exercise Physiology. 2017;6(3):103-10. [View at Publisher] [DOI] [Google Scholar]
24. Takahashi M, Suzuki K, Matoba H, Sakamoto S, Obara S. Effects of different intensities of endurance exercise on oxidative stress and antioxidant capacity. The Journal of Physical Fitness and Sports Medicine. 2012;1(1):183-9. [View at Publisher] [DOI] [Google Scholar]
25. Fico BG, Slusher AL, Whitehurst M, Maharaj A, Huang C-J. The Impact of Obesity on Calprotectin Response to Acute Aerobic Exercise: 2661 Board# 184 June 3, 9: 30 AM-11: 00 AM. Medicine & Science in Sports & Exercise. 2016;48(5S)(suppl1):743. [View at Publisher] [DOI]
26. Calcaterra V, De Amici M, Leonard MM, De Silvestri A, Pelizzo G, Buttari N, et al. Serum calprotectin level in children: marker of obesity and its metabolic complications. Ann Nutr Metab. 2018;73(3):177-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Niemelä M, Niemelä O, Bloigu R, Bloigu A, Kangastupa P, Juvonen T. Serum calprotectin, a marker of neutrophil activation, and other mediators of inflammation in response to various types of extreme physical exertion in healthy volunteers. J Inflamm Res. 2020;13:223-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Grand A, Rochette E, Dutheil F, Gozal D, Calcaterra V, Berni Canani R, et al. Body mass index and calprotectin blood level correlation in healthy children: an individual patient data meta-analysis. J Clin Med. 2020;9(3):857. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Talebi-Garakani E, Vosough M, Kheradmand S, Fathi R. The effect of eight weeks aerobic exercise training on plasma levels of Calprotectin and insulin resistance in overweight post-menopausal and peri-menopausal women. Journal of Practical Studies of Biosciences in Sport. 2021;9(20):56-66. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





