Volume 11, Issue 1 (7-2023)
Jorjani Biomed J 2023, 11(1): 9-12 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jadidi S, Avandi S M, Khaleghian A. Effects of high-intensity functional training and circuit resistance training on the serum levels of IFN-γ in obese women. Jorjani Biomed J 2023; 11 (1) :9-12
URL: http://goums.ac.ir/jorjanijournal/article-1-945-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-945-en.html
1- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,m.avandi@semnan.ac.ir
3- Biochemistry Department, Semnan University of Medical sciences, Semnan, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,
3- Biochemistry Department, Semnan University of Medical sciences, Semnan, Iran
Keywords: High-intensity functional training, Circuit resistance training, Interferon gamma, Obesity
Full-Text [PDF 574 kb]
(2868 Downloads)
| Abstract (HTML) (9179 Views)
Full-Text: (1670 Views)
Introduction
Obesity is a severe health issue today associated with various diseases, such as cardiovascular and metabolic disorders, atherosclerosis, type II diabetes, and metabolic syndrome (1). Obesity is also associated with chronic systemic inflammation, which increases the production and release of pro-inflammatory cytokines (2).
Interferon-gamma (IFN-γ) is an important pro-inflammatory cytokine that regulates the systemic inflammatory process (2). Resistance training is one of the main sports training protocols, positively affecting various body organs (3). Few studies have examined the effect of resistance training on inflammation, indicating that this training is associated with reduced inflammation risk and improved metabolic diseases (4).
A study showed that ten weeks of moderate-to-severe resistance training inhibited inflammatory markers in older women (5). Furthermore, eight weeks of resistance training has been associated with an increase in some anti-inflammatory agents in obese women (6). In another study, resistance training increased IL-4 and IL-6 and decreased IFN-γ (7).
High-intensity functional training (HIFT) is a relatively new resistance training method that emphasizes functional and multi-joint exercises that significantly improve cardiovascular endurance, strength, and maximum oxygen consumption (8). Recently, a HIFT program was reported to be effective in improving cardiorespiratory and neuromuscular fitness without significant inflammation. Additionally, the HIFT program reported a decrease in the body fat percentage of active individuals (9). Therefore, HIFT may be a potentially useful strategy to combat obesity and inflammation.
Circuit resistance training (CRT) is another type of resistance training, a popular training intervention that could increase anti-inflammatory markers and improve body composition in obese individuals (10).
Despite limited studies on the effects of resistance training on inflammation and the potential mechanisms of the training effects on obesity-related inflammation, no findings are available to compare the effects of HIFT and circuit exercises on the IFN-γ in obese women. The present study aimed to evaluate the effects of six weeks of HIFT and CRT on the serum levels of IFN-γ in obese women.
Methods
Participants
This quasi-experimental study was conducted with a pretest-posttest design on two experimental and control groups. The sample size was calculated according to previous research, and in total, thirty-six obese women aged 20-40 years were selected according to the inclusion and exclusion criteria. The participants were randomly divided into two experimental groups (n=12 in the HIFT group, n=12 in the CRT group) and a control group (n=12).
The inclusion criteria of this study were as follows: 1) no chronic diseases, injuries, or conditions limiting participation in physical activities; 2) body mass index (BMI) of >30 kg/m2; 3) no history of sleep disorders; 4) no smoking habits; 5) no use of supplements, alcohol, and caffeinated substances; 6) not receiving medication therapy. The exclusion criteria were as follows: 1) absence of more than one session in the exercise intervention; 2) occurrence of accidents or injuries; 3) knee disorders; 4) presence of interfering factors in active participation in the training program.
Initially, the participants were invited to the laboratory location, and demographic data were collected on their age, height, weight, and BMI. Afterwards, the subjects of the intervention groups implemented the desired protocol. Blood samples were obtained before the intervention after 12 hours of fasting and 48 hours since the last training session to measure the serum levels of IFN-γ.
Training Protocols
The participants were introduced to the correct technique of exercise, breathing techniques, and performing the exercises in a gym under the supervision of an instructor before starting the intervention. Before performing circuit resistance exercises, the participants had a 10-minute warm-up (stretching and flexibility). The circuit resistance protocol included arm curl, triceps push-down, chest press, rowing, abdominal crunches, leg press, and leg curl exercise. The exercises were performed in three weekly sessions with 40-50% of one repetition maximum (Table 1) (11). At the beginning of training and in the fourth and sixth weeks, maximum strength was measured using the one maximum repetition test (2).

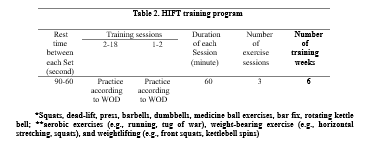
HIFT protocol was performed in line with the study by Feito et al. The total training period was 18 sessions (six weeks), and each session was approximately 60 minutes. The HIFT sessions were supervised by a resistance training instructor (Table 2) (12).
Post-test blood samples were obtained to measure biochemical variables after 12-14 hours of fasting and 48 hours before the last training session. For this purpose, 11 milliliters of venous blood were collected from the brachial vein after five minutes of rest. The blood samples were centrifuged at 1,500 rounds for 15 minutes to prepare the serum, and the obtained serum was preserved at -80°C for further analysis. The IFN-γ inflammatory agent was measured using the Human IFN-g Elisa kit (model: 96t-zellbio, made in Germany).
Data analysis was performed in SPSS version 24 using the Shapiro-Wilk test to determine the natural distribution of data among the groups. In addition, Levene’s test was used to assess the homogeneity of variance between the groups. One-way analysis of variance (ANOVA) and dependent t-test were also applied at the significance level of P≤0.05. The null hypothesis of the research was investigated with a probability of error of P≤0.05.
Results
Table 3 shows the anthropometric indices of the subjects before and after six weeks of training. The group results showed no significant difference in weight, waist-to-hip ratio, or BMI between the HIFT and CRT training groups (P>0.05). In addition, the inter-group results showed no significant change in the weight, waist-to-hip ratio, and BMI between the training and control groups (P>0.05) (Table 3).

serum IFN-γ levels (P>0.05). In addition, there was no significant difference in serum IFN-γ levels between the training and control groups (P>0.05) (Figure 1 and Table 4).


Discussion
According to the intragroup and intergroup results of the present study, six weeks of HIFT and CRT had no significant effects on the IFN-γ levels of obese women. Weight and fat gain are associated with the accumulation of macrophages in the adipose tissue and the increased expression of pro-inflammatory proteins. Therefore, the inflammatory profile of the adipose tissue increases in obese individuals (2). Activated macrophages release pro-inflammatory cytokines significantly associated with body mass (13). IFN-γ is one of these pro-inflammatory cytokines, which increases in the setting of systemic inflammation due to obesity (2). Increased pro-inflammatory markers in the adipose tissue of obese individuals increase insulin resistance, endothelial dysfunction, and atherosclerosis (14).
A growing interest exists in using lifestyle-related interventions (e.g., increased physical activity) to reduce chronic inflammation and the risk of various diseases. In this regard, the results obtained by Petersen et al. (2005), Brinkley & Nicklas (2009), and Lavie et al. (2011) confirmed the positive effects of exercise on chronic inflammation (15-17). However, the specific effects of exercise training on chronic obesity inflammation and the potential underlying mechanisms require further investigation (18). Limited studies have focused on the effect of resistance training on the serum levels of inflammation-related IFN-γ, and no research has used HIFT and CRT regarding their impact on IFN-γ. In a study conducted by Larsen et al. (2001), no changes were reported in the levels of IFN-γ during and after resistance training, which is consistent with the results of the present study (19). On the other hand, White et al. (2006) reported that performing an eight-week resistance training course could reduce IFN-γ levels at rest (20). In addition, Pour et al. (2015) and Nurtjahja et al. (2003) reported that a 16-week resistance training intervention could decrease pro-inflammatory cytokines such as IFN-γ (7, 21). Furthermore, Phillips et al. (2012) stated that 18 weeks of moderate-to-severe resistance training reduced inflammatory markers in active women (22). On the other hand, Libardi et al. (2012) reported that 16 weeks of resistance training had no significant effects on the inflammatory markers of healthy middle-aged men (23).
The current research findings demonstrated that CRT and HIFT had no significant effects on the anthropometric indices of obese women. According to the literature, the levels of inflammatory markers are often higher in obese individuals than those with normal weight. Accordingly, weight loss in obese subjects could decrease the levels of some inflammatory markers (24). The accumulation of macrophages in the adipose tissue is proportional to the degree of obesity (25). In the present study, CRT and HIFT had no significant effects on weight loss and fat percentage. Therefore, it could be concluded that the ineffectiveness of these exercises in decreasing the serum levels of IFN-γ was associated with no weight loss and the body composition of obese women.
Seemingly, various responses of inflammatory indicators in previous studies are due to differences in the implemented exercise programs and training protocols, participants’ training history, duration and intensity of training, exercise habits, and adaptation to exercise (7,26). Similarly, Jeurissen et al. (2003) confirmed this claim by stating that physical activity has varying effects on the human immune system depending on its duration and intensity (27). For instance, gentle, continuous, and regular exercise increases the body’s resistance to infections (e.g., upper respiratory tract infections) by boosting the immune system. On the other hand, intense exercise significantly reduces the body’s resistance to such infections (28) and increases pro-inflammatory cytokines. In this regard, Pedersen et al. (2000) stated that the presence of several cytokines in the urine after intense exercise indicated the expression of cytokines in response to exercise (29).
In two other studies, strenuous exercise was shown to increase the number of various stress inflammatory cells, such as lymphocytes (T, B, and NK), monocytes, and neutrophils, all of which could produce a wide range of cytokines, secreting and releasing growth factors (30, 31). In addition, Fish et al. (2003) reported that physical activity has a dual effect on inflammation, and intense and prolonged physical activity increases inflammation and infection (32).
Jahromi Sotoudeh et al. (2014) showed that endurance training significantly affected the serum levels of IFN-γ in sedentary men (33). The results of the present study in this regard were not considered significant in the experimental groups. However, HIFT is an effective strategy in maintaining interest and pleasure in sedentary individuals (8) and seems to be a potentially beneficial strategy in battling obesity and inflammation (34).
Limitation
One of the essential limitations of the current research was the spread of the coronavirus, the loss of samples, and the shortness of the training period. It is suggested that the same exercise protocol should be performed over a longer period and with more subjects in future research.
Conclusion
The results of the present study showed that HIFT and CRT did not reduce the serum levels of IFN-γ in obese women. Given that this study was the first to compare the effects of circuit resistance training and HIFT and data are scarce, further investigation is required to reach a definitive conclusion.
Ethical Statement
Researchers received introduction letters from Semnan University of Medical Sciences of Iran with the code: IR.SEMUMS.REC.1399.107
Acknowledgments
Hereby, the authors extend their gratitude to all participants for assisting them in compiling, implementing, and completing this research project.
Conflict of interest
The authors have no conflict of interest that are directly relevant to the content of this original research paper.
Obesity is a severe health issue today associated with various diseases, such as cardiovascular and metabolic disorders, atherosclerosis, type II diabetes, and metabolic syndrome (1). Obesity is also associated with chronic systemic inflammation, which increases the production and release of pro-inflammatory cytokines (2).
Interferon-gamma (IFN-γ) is an important pro-inflammatory cytokine that regulates the systemic inflammatory process (2). Resistance training is one of the main sports training protocols, positively affecting various body organs (3). Few studies have examined the effect of resistance training on inflammation, indicating that this training is associated with reduced inflammation risk and improved metabolic diseases (4).
A study showed that ten weeks of moderate-to-severe resistance training inhibited inflammatory markers in older women (5). Furthermore, eight weeks of resistance training has been associated with an increase in some anti-inflammatory agents in obese women (6). In another study, resistance training increased IL-4 and IL-6 and decreased IFN-γ (7).
High-intensity functional training (HIFT) is a relatively new resistance training method that emphasizes functional and multi-joint exercises that significantly improve cardiovascular endurance, strength, and maximum oxygen consumption (8). Recently, a HIFT program was reported to be effective in improving cardiorespiratory and neuromuscular fitness without significant inflammation. Additionally, the HIFT program reported a decrease in the body fat percentage of active individuals (9). Therefore, HIFT may be a potentially useful strategy to combat obesity and inflammation.
Circuit resistance training (CRT) is another type of resistance training, a popular training intervention that could increase anti-inflammatory markers and improve body composition in obese individuals (10).
Despite limited studies on the effects of resistance training on inflammation and the potential mechanisms of the training effects on obesity-related inflammation, no findings are available to compare the effects of HIFT and circuit exercises on the IFN-γ in obese women. The present study aimed to evaluate the effects of six weeks of HIFT and CRT on the serum levels of IFN-γ in obese women.
Methods
Participants
This quasi-experimental study was conducted with a pretest-posttest design on two experimental and control groups. The sample size was calculated according to previous research, and in total, thirty-six obese women aged 20-40 years were selected according to the inclusion and exclusion criteria. The participants were randomly divided into two experimental groups (n=12 in the HIFT group, n=12 in the CRT group) and a control group (n=12).
The inclusion criteria of this study were as follows: 1) no chronic diseases, injuries, or conditions limiting participation in physical activities; 2) body mass index (BMI) of >30 kg/m2; 3) no history of sleep disorders; 4) no smoking habits; 5) no use of supplements, alcohol, and caffeinated substances; 6) not receiving medication therapy. The exclusion criteria were as follows: 1) absence of more than one session in the exercise intervention; 2) occurrence of accidents or injuries; 3) knee disorders; 4) presence of interfering factors in active participation in the training program.
Initially, the participants were invited to the laboratory location, and demographic data were collected on their age, height, weight, and BMI. Afterwards, the subjects of the intervention groups implemented the desired protocol. Blood samples were obtained before the intervention after 12 hours of fasting and 48 hours since the last training session to measure the serum levels of IFN-γ.
Training Protocols
The participants were introduced to the correct technique of exercise, breathing techniques, and performing the exercises in a gym under the supervision of an instructor before starting the intervention. Before performing circuit resistance exercises, the participants had a 10-minute warm-up (stretching and flexibility). The circuit resistance protocol included arm curl, triceps push-down, chest press, rowing, abdominal crunches, leg press, and leg curl exercise. The exercises were performed in three weekly sessions with 40-50% of one repetition maximum (Table 1) (11). At the beginning of training and in the fourth and sixth weeks, maximum strength was measured using the one maximum repetition test (2).

HIFT protocol was performed in line with the study by Feito et al. The total training period was 18 sessions (six weeks), and each session was approximately 60 minutes. The HIFT sessions were supervised by a resistance training instructor (Table 2) (12).

Post-test blood samples were obtained to measure biochemical variables after 12-14 hours of fasting and 48 hours before the last training session. For this purpose, 11 milliliters of venous blood were collected from the brachial vein after five minutes of rest. The blood samples were centrifuged at 1,500 rounds for 15 minutes to prepare the serum, and the obtained serum was preserved at -80°C for further analysis. The IFN-γ inflammatory agent was measured using the Human IFN-g Elisa kit (model: 96t-zellbio, made in Germany).
Data analysis was performed in SPSS version 24 using the Shapiro-Wilk test to determine the natural distribution of data among the groups. In addition, Levene’s test was used to assess the homogeneity of variance between the groups. One-way analysis of variance (ANOVA) and dependent t-test were also applied at the significance level of P≤0.05. The null hypothesis of the research was investigated with a probability of error of P≤0.05.
Results
Table 3 shows the anthropometric indices of the subjects before and after six weeks of training. The group results showed no significant difference in weight, waist-to-hip ratio, or BMI between the HIFT and CRT training groups (P>0.05). In addition, the inter-group results showed no significant change in the weight, waist-to-hip ratio, and BMI between the training and control groups (P>0.05) (Table 3).

serum IFN-γ levels (P>0.05). In addition, there was no significant difference in serum IFN-γ levels between the training and control groups (P>0.05) (Figure 1 and Table 4).


Discussion
According to the intragroup and intergroup results of the present study, six weeks of HIFT and CRT had no significant effects on the IFN-γ levels of obese women. Weight and fat gain are associated with the accumulation of macrophages in the adipose tissue and the increased expression of pro-inflammatory proteins. Therefore, the inflammatory profile of the adipose tissue increases in obese individuals (2). Activated macrophages release pro-inflammatory cytokines significantly associated with body mass (13). IFN-γ is one of these pro-inflammatory cytokines, which increases in the setting of systemic inflammation due to obesity (2). Increased pro-inflammatory markers in the adipose tissue of obese individuals increase insulin resistance, endothelial dysfunction, and atherosclerosis (14).
A growing interest exists in using lifestyle-related interventions (e.g., increased physical activity) to reduce chronic inflammation and the risk of various diseases. In this regard, the results obtained by Petersen et al. (2005), Brinkley & Nicklas (2009), and Lavie et al. (2011) confirmed the positive effects of exercise on chronic inflammation (15-17). However, the specific effects of exercise training on chronic obesity inflammation and the potential underlying mechanisms require further investigation (18). Limited studies have focused on the effect of resistance training on the serum levels of inflammation-related IFN-γ, and no research has used HIFT and CRT regarding their impact on IFN-γ. In a study conducted by Larsen et al. (2001), no changes were reported in the levels of IFN-γ during and after resistance training, which is consistent with the results of the present study (19). On the other hand, White et al. (2006) reported that performing an eight-week resistance training course could reduce IFN-γ levels at rest (20). In addition, Pour et al. (2015) and Nurtjahja et al. (2003) reported that a 16-week resistance training intervention could decrease pro-inflammatory cytokines such as IFN-γ (7, 21). Furthermore, Phillips et al. (2012) stated that 18 weeks of moderate-to-severe resistance training reduced inflammatory markers in active women (22). On the other hand, Libardi et al. (2012) reported that 16 weeks of resistance training had no significant effects on the inflammatory markers of healthy middle-aged men (23).
The current research findings demonstrated that CRT and HIFT had no significant effects on the anthropometric indices of obese women. According to the literature, the levels of inflammatory markers are often higher in obese individuals than those with normal weight. Accordingly, weight loss in obese subjects could decrease the levels of some inflammatory markers (24). The accumulation of macrophages in the adipose tissue is proportional to the degree of obesity (25). In the present study, CRT and HIFT had no significant effects on weight loss and fat percentage. Therefore, it could be concluded that the ineffectiveness of these exercises in decreasing the serum levels of IFN-γ was associated with no weight loss and the body composition of obese women.
Seemingly, various responses of inflammatory indicators in previous studies are due to differences in the implemented exercise programs and training protocols, participants’ training history, duration and intensity of training, exercise habits, and adaptation to exercise (7,26). Similarly, Jeurissen et al. (2003) confirmed this claim by stating that physical activity has varying effects on the human immune system depending on its duration and intensity (27). For instance, gentle, continuous, and regular exercise increases the body’s resistance to infections (e.g., upper respiratory tract infections) by boosting the immune system. On the other hand, intense exercise significantly reduces the body’s resistance to such infections (28) and increases pro-inflammatory cytokines. In this regard, Pedersen et al. (2000) stated that the presence of several cytokines in the urine after intense exercise indicated the expression of cytokines in response to exercise (29).
In two other studies, strenuous exercise was shown to increase the number of various stress inflammatory cells, such as lymphocytes (T, B, and NK), monocytes, and neutrophils, all of which could produce a wide range of cytokines, secreting and releasing growth factors (30, 31). In addition, Fish et al. (2003) reported that physical activity has a dual effect on inflammation, and intense and prolonged physical activity increases inflammation and infection (32).
Jahromi Sotoudeh et al. (2014) showed that endurance training significantly affected the serum levels of IFN-γ in sedentary men (33). The results of the present study in this regard were not considered significant in the experimental groups. However, HIFT is an effective strategy in maintaining interest and pleasure in sedentary individuals (8) and seems to be a potentially beneficial strategy in battling obesity and inflammation (34).
Limitation
One of the essential limitations of the current research was the spread of the coronavirus, the loss of samples, and the shortness of the training period. It is suggested that the same exercise protocol should be performed over a longer period and with more subjects in future research.
Conclusion
The results of the present study showed that HIFT and CRT did not reduce the serum levels of IFN-γ in obese women. Given that this study was the first to compare the effects of circuit resistance training and HIFT and data are scarce, further investigation is required to reach a definitive conclusion.
Ethical Statement
Researchers received introduction letters from Semnan University of Medical Sciences of Iran with the code: IR.SEMUMS.REC.1399.107
Acknowledgments
Hereby, the authors extend their gratitude to all participants for assisting them in compiling, implementing, and completing this research project.
Conflict of interest
The authors have no conflict of interest that are directly relevant to the content of this original research paper.
Type of Article: Original article |
Subject:
Health
Received: 2022/12/29 | Accepted: 2023/07/15 | Published: 2023/07/1
Received: 2022/12/29 | Accepted: 2023/07/15 | Published: 2023/07/1
References
1. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71-82. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Khazaei M, Rouzbahani R. Obesity and inflammation: Role of adipokines. Journal of Isfahan Medical School (JIMS). 2016;34(372):148-56. [View at Publisher] [Google scholar]
3. Fleck SJ, Kraemer W. Designing resistance training programs, 4E. United States: Human Kinetics; 2014. [View at Publisher] [DOI] [PMID] [Google scholar]
4. Calle MC, Fernandez ML. Effects of resistance training on the inflammatory response. Nutr Res Pract. 2010;4(4):259-69. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc. 2010;42(2):314-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Kalhor F, Arshadi S, Zafari A. The effect the period of a resistance training on Atrogin, eotaxin and IL-10 indices in obese women. Razi Journal of Medical Sciences. 2020;27(3):130-7. [View at Publisher] [Google scholar]
7. Pour MD, Naghibi M. Effects of 2 Types of Resistance Training, Pyramid and Reverse pyramid training, on IL-4, IL-6, and IFN-γ in Young women. Biomedical and Pharmacology Journal. 2015;8(2):915-21. [View at Publisher] [DOI] [Google scholar]
8. Heinrich KM, Becker C, Carlisle T, Gilmore K, Hauser J, Frye J, et al. High‐intensity functional training improves functional exercise and body composition among cancer survivors: a pilot study. Eur J Cancer Care (Engl). 2015;24(6):812-7. [View at Publisher] [DOI] [PMID] [Google scholar]
9. Posnakidis G, Aphamis G, Giannaki CD, Mougios V, Aristotelous P, Samoutis G, et al. High-Intensity Functional Training Improves Cardiorespiratory Fitness and Neuromuscular Performance Without Inflammation or Muscle Damage. J Strength Cond Res. 2022;36(3):615-23. [View at Publisher] [DOI] [PMID] [Google scholar]
10. Nakhaei K, Ghofrani M, Fazel Bakhsheshi M, Nakhaei H. Effect of circuit resistance training and cinnamon supplement on body composition and Omentin-1 in overweight women. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2018;20(11):74-82. [View at Publisher] [DOI] [Google scholar]
11. Rezaeian N, Ravasi AA, Soori R, Akbarnezhad A, Mirshafiey SA, Towfighi F. Effect of Resistance Training on Serum Levels of Adipolin and Insulin Resistance in Obese Women. Journal of Sport Biosciences. 2020;12(1):1-16. [View at Publisher] [DOI] [Google scholar]
12. Feito Y, Heinrich KM, Butcher SJ, Poston WSC. High-intensity functional training (HIFT): definition and research implications for improved fitness. Sports (Basel). 2018;6(3):76. [View at Publisher] [DOI] [PMID] [Google scholar]
13. Christiansen T, Richelsen B, Bruun J. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond). 2005;29(1):146-50. [View at Publisher] [DOI] [PMID] [Google scholar]
14. Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144(6):2195-200. [View at Publisher] [DOI] [PMID] [Google scholar]
15. Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. Journal of applied physiology. 2005;98(4):1154-62. [View at Publisher] [DOI] [PMID] [Google scholar]
16. Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exercise and sport sciences reviews. 2009;37(4):165-70. [View at Publisher] [DOI] [PMID] [Google scholar]
17. Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31(3):137-45. [View at Publisher] [DOI] [PMID] [Google scholar]
18. You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity. Sports Med. 2013;43(4):243-56. [View at Publisher] [DOI] [PMID] [Google scholar]
19. Larsen AI, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88(7):805-8. [View at Publisher] [DOI] [PMID] [Google scholar]
20. White LJ, Castellano V, Mc Coy SC. Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci. 2006;24(8):911-4. [View at Publisher] [DOI] [PMID] [Google scholar]
21. Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111(4-5):259-65. [View at Publisher] [DOI] [PMID] [Google scholar]
22. Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44(11):2099-110. [View at Publisher] [DOI] [PMID] [Google scholar]
23. Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MPT. Effect of resistance, endurance, and concurrent training on TNF-α, IL-6, and CRP. Med Sci Sports Exerc. 2012;44(1):50-6. [View at Publisher] [DOI] [PMID] [Google scholar]
24. 24 Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91. [View at Publisher] [DOI] [PMID] [Google scholar]
25. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112(12):1796-808. [View at Publisher] [DOI] [PMID] [Google scholar]
26. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277-86. [View at Publisher] [DOI] [PMID] [Google scholar]
27. Jeurissen A, Bossuyt X, Ceuppens J, Hespel P. The effects of physical exercise on the immune system. Nederlands tijdschrift voor geneeskunde. 2003;147(28):1347-51. [View at Publisher] [PMID] [Google scholar]
28. Gleeson M. Biochemical and immunological markers of over-training. J Sports Sci Med. 2002;1(2):31-41. [View at Publisher] [PMID] [Google scholar]
29. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055-81. [View at Publisher] [DOI] [PMID] [Google scholar]
30. Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003;33(4):261-84. [View at Publisher] [DOI] [PMID] [Google scholar]
31. Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJV, Drayson MT, et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23(6):767-75. [View at Publisher] [DOI] [PMID] [Google scholar]
32. Fish DE, Krabak BJ, Johnson-Greene D, DeLateur BJ. Optimal resistance training: comparison of DeLorme with Oxford techniques. Am J Phys Med Rehabil. 2003;82(12):903-9. [View at Publisher] [DOI] [PMID] [Google scholar]
33. Jahromi AS, Zar A, Ahmadi F, Krustrup P, Ebrahim K, Hovanloo F, et al. Effects of endurance training on the serum levels of tumour necrosis factor-α and interferon-γ in sedentary men. Immune Netw. 2014;14(5):255-9. [View at Publisher] [DOI] [PMID] [Google scholar]
34. Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32(6):584-8. [View at Publisher] [DOI] [PMID] [Google scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





