Volume 10, Issue 1 (3-2022)
Jorjani Biomed J 2022, 10(1): 67-83 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Behboudi E, Zeynali P, Zahedian Nezhad N, Hamidi Sofiani V. Vitamin A and Viral Infection in Critical Care. Jorjani Biomed J 2022; 10 (1) :67-83
URL: http://goums.ac.ir/jorjanijournal/article-1-876-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-876-en.html
1- Department of Microbiology, Golestan University of Medical Sciences, Gorgan, Iran
2- Department of Biochemistry and Biophysics, Metabolic Disorders Research Center, School of Medicine, Golestan University of Medical Science, Gorgan, Iran
3- Department of Microbiology, Golestan University of Medical Sciences, Gorgan, Iran , hamidi.v@goums.ac.ir
2- Department of Biochemistry and Biophysics, Metabolic Disorders Research Center, School of Medicine, Golestan University of Medical Science, Gorgan, Iran
3- Department of Microbiology, Golestan University of Medical Sciences, Gorgan, Iran , hamidi.v@goums.ac.ir
Full-Text [PDF 942 kb]
(1194 Downloads)
| Abstract (HTML) (4506 Views)
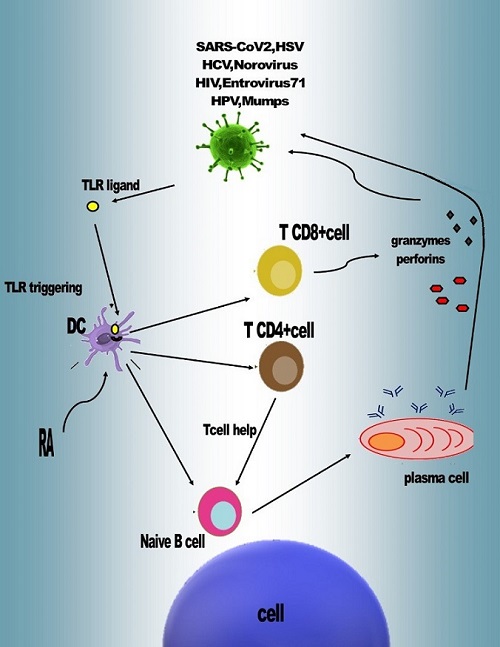
Vitamin A has direct and indirect effects in modulating immune responses
Vitamin A deficiency is involved in the severity of different viral infections
Vitamin A is an important element in cytokine storm
.png)

8. Mumps
U937 cells are considered as neoplastic and histiocytic progenitors of monocytes that in many times during immunological studies have been used (132). These cells specially are an important case during Mumps virus (MuV) infection to studying of interferon pathways(133-131). During studies get understood that increasing doses of retinol in these cells act as an inhibitor for MuV replication and the quantity of process measured by TCID50. It was observed that concentrations as low as 1 μM, act as suitable inhibitor and also with this dose increased expression of the retinoid responsive gene RARβ was observed. Increasing doses of ATRA during treatment of U937 cells have efficient operation as inhibitor of MuV output and are effective in the induction of RARβ mRNA expression (132). Several studies reported ATRA at a dose of 1 μM have antiviral effect on MuV. Also IFN signaling increases during the Retinoid treatment. As a primary control of infection, the innate immune response thought to be responsible for immunity and it is mentioned that up-regulation of the type I IFN response functions have an important role in antiviral responses. When MuV infection alone occurred in the U937 model, it causes induction in expression of IFNα1 mRNA. Also, using ATRA as a treatment of MuV infected cells act as a cofactor and its operation has a synergism action to increase the expression of IFNα1 mRNA and its protein levels. So with these concepts it can be assumed that expression of IFNβ mRNA and its protein levels, during treatment of ATRA in MuV infection will get increased. The expression of ISGs will be influenced by increasing in type I IFN production. Also, over the treatment by ATRA in the U937 model, (106) IRF-1 mRNA expression get increased, and this result is favorable in some previous studies and literatures (108, 133, 134). So for expression of RIG-I mRNA, treatment with ATRA is required (Table1) (132).
Conclusion
The promising role of vitamin A in different viral infections is well‐described by several epidemiological studies, supporting the notion that higher level of vitamin A is associated with better prognosis and improved outcomes. Although the mechanisms responsible for vitamin A function in the host immune system have been widely described, the interplay between viral infections and vitamin A status remains an intriguing area, and the potential interactions between viral infections and vitamin A appears to be more complex than our previous knowledge. Induction of antimicrobial peptides, immunoregulatory function, interaction with cellular and viral factors are the main underlying mechanisms by which vitamin A insufficiency could contribute to viral disease development. These Data demonstrate that retinoid can inhibit viral replication commonly through a retinoid inducible gene I (RIG-I), Retinoic Acid Receptor (RAR) and IFN dependent manner making them refractory to subsequent rounds of viral replication. These observations raise the possibility that pharmacological doses of retinoids might have clinical benefit in different viral infections.
Declaration
This study has been conducted in Microbiology Department of the School of Medicine Golestan University of Medical Sciences.
Conflict of interest
The authors declare no conflicts of interests.

Vitamin A has direct and indirect effects in modulating immune responses
Vitamin A deficiency is involved in the severity of different viral infections
Vitamin A is an important element in cytokine storm
Full-Text: (194 Views)
Introduction
Vitamin A (VA) has been known as a critical fat-soluble vitamin that is needed for human health (1, 2). It is a micronutrient in diet, which is necessary for the immune system and facilitates modulation of Retinoic acid (RA) (3, 4). Also this vitamin plays a key role in growth and fertility mechanisms (5). Its functional form in the body has been recognized as All Trans’ Retinoic Acid (ATRA)(5-7). Uptaking vitamin A from meat occurs as retinyl esters of fatty acids in membranous lipid and fat cells. Carotenoids are Pro-vitamin A in vegetables that are connected with cellular lipids. However, they are located in cellular complex structures like the chloroplasts cellulose-consisting matrix. Studies on vitamin A and carotenoid absorption in digestion procedure from embedding food has shown that better absorption of micronutrients occurs from meat toward vegetable tissues (2, 5, 8-11). Various metabolites of vitamin A regulate proliferation, differentiation, cell death of epithelial cells, penetrability of the intestinal epithelium, and immunity tasks of the gastrointestinal epithelium(12-14). The vitamin A supplements decreases rate of morbidity and mortality in measles (15, 16), diarrhea (17, 18), respiratory tract infections (17, 19, 20), malaria (21, 22), HIV infection (23, 24) and some other infections (25, 26). Furthermore, some impacts of vitamin A on immune system elements toward pathogens have been recognized by medical scientists in clinical studies. Numerous reports, have shown the extensive effect of vitamin A and its other metabolites on the expansion and function of the immunity agents in children, consisting of its special effects on T (27) and B-cells, antigen-presenting cells (APCs), dendritic cells (DCs) (28), and other immune system elements (26- 28). On the other hand, vitamin A deficiency (VAD) is linked with decreased immunogenicity of various vaccines (29) including diphtheria, measles (26, 30) and tetanus toxoid (31). Retinoic acid has known to facilitate IgA production and gut homing of IgA+ plasma cells (32) and T-cells (33). Besides mediating these responses, vitamin A is also crucial for regular T-cell responses in the intestinal mucosa (29, 33- 35). Therefore, vitamin A is involved in modulation of intestinal immunity. Vitamin A deficiency shifts cytokine responses as polarization to amplified T-helper cell type 1 (Th1) cytokine responses in children (36). Some studies revealed that treatment of T cells with RA enhances Th2 development and decreases Th1 production (37, 38). These comparisons suggest that the modulation of immune function by vitamin A is complex and involves many different arms of the immune system (5, 39). Interestingly, retinol upregulates IgA production (1) in vitro when solenocyte B cells are triggered in the presence of a respiratory epithelial cell line (LETs) (40), in an IL-6 dependent manner (25, 41). Despite its clear positive influences on IgA induction, modulation of immune responses through vitamin A and its active metabolites has been shown to occur via nuclear retinoic acid receptors at the level of the genome as well as via a pathway involving retroretinoids (42, 43). Even though vitamin A and its metabolites have been demonstrated to have key unspecific effects on host immunity, the therapeutic property of vitamin A intake in improving differentiation to various types of viral agents differ significantly (26, 42). The purpose of the present paper is to gain an insight into the effects of vitamin A in modulating immune responses in viral infections and direct effects of this vitamin on viral reproduction by comparing its function during different types of viral infections.
Materials and Methods
Literature search strategy
This systematic review explored the advantages of vitamin A administration in different viral infections. The Embase, PubMed, and Scopus databases were searched up to July 22keywords single and combinations of the following key words: (Vitamin A and Viral and infection and viruses) and (Vitamin A OR Viral OR infection OR viruses). The search strategy for this review was conducted as the (Fig-1. Flowchart).
Study selection
Briefly, the extracted articles were screened by two independent reviewers and based on the titles and abstracts, some of articles were excluded. Then articles without full text were excluded.
Vitamin A (VA) has been known as a critical fat-soluble vitamin that is needed for human health (1, 2). It is a micronutrient in diet, which is necessary for the immune system and facilitates modulation of Retinoic acid (RA) (3, 4). Also this vitamin plays a key role in growth and fertility mechanisms (5). Its functional form in the body has been recognized as All Trans’ Retinoic Acid (ATRA)(5-7). Uptaking vitamin A from meat occurs as retinyl esters of fatty acids in membranous lipid and fat cells. Carotenoids are Pro-vitamin A in vegetables that are connected with cellular lipids. However, they are located in cellular complex structures like the chloroplasts cellulose-consisting matrix. Studies on vitamin A and carotenoid absorption in digestion procedure from embedding food has shown that better absorption of micronutrients occurs from meat toward vegetable tissues (2, 5, 8-11). Various metabolites of vitamin A regulate proliferation, differentiation, cell death of epithelial cells, penetrability of the intestinal epithelium, and immunity tasks of the gastrointestinal epithelium(12-14). The vitamin A supplements decreases rate of morbidity and mortality in measles (15, 16), diarrhea (17, 18), respiratory tract infections (17, 19, 20), malaria (21, 22), HIV infection (23, 24) and some other infections (25, 26). Furthermore, some impacts of vitamin A on immune system elements toward pathogens have been recognized by medical scientists in clinical studies. Numerous reports, have shown the extensive effect of vitamin A and its other metabolites on the expansion and function of the immunity agents in children, consisting of its special effects on T (27) and B-cells, antigen-presenting cells (APCs), dendritic cells (DCs) (28), and other immune system elements (26- 28). On the other hand, vitamin A deficiency (VAD) is linked with decreased immunogenicity of various vaccines (29) including diphtheria, measles (26, 30) and tetanus toxoid (31). Retinoic acid has known to facilitate IgA production and gut homing of IgA+ plasma cells (32) and T-cells (33). Besides mediating these responses, vitamin A is also crucial for regular T-cell responses in the intestinal mucosa (29, 33- 35). Therefore, vitamin A is involved in modulation of intestinal immunity. Vitamin A deficiency shifts cytokine responses as polarization to amplified T-helper cell type 1 (Th1) cytokine responses in children (36). Some studies revealed that treatment of T cells with RA enhances Th2 development and decreases Th1 production (37, 38). These comparisons suggest that the modulation of immune function by vitamin A is complex and involves many different arms of the immune system (5, 39). Interestingly, retinol upregulates IgA production (1) in vitro when solenocyte B cells are triggered in the presence of a respiratory epithelial cell line (LETs) (40), in an IL-6 dependent manner (25, 41). Despite its clear positive influences on IgA induction, modulation of immune responses through vitamin A and its active metabolites has been shown to occur via nuclear retinoic acid receptors at the level of the genome as well as via a pathway involving retroretinoids (42, 43). Even though vitamin A and its metabolites have been demonstrated to have key unspecific effects on host immunity, the therapeutic property of vitamin A intake in improving differentiation to various types of viral agents differ significantly (26, 42). The purpose of the present paper is to gain an insight into the effects of vitamin A in modulating immune responses in viral infections and direct effects of this vitamin on viral reproduction by comparing its function during different types of viral infections.
Materials and Methods
Literature search strategy
This systematic review explored the advantages of vitamin A administration in different viral infections. The Embase, PubMed, and Scopus databases were searched up to July 22keywords single and combinations of the following key words: (Vitamin A and Viral and infection and viruses) and (Vitamin A OR Viral OR infection OR viruses). The search strategy for this review was conducted as the (Fig-1. Flowchart).
Study selection
Briefly, the extracted articles were screened by two independent reviewers and based on the titles and abstracts, some of articles were excluded. Then articles without full text were excluded.
Diagram1. Flowchart of systematic review
.png)
Literature Search
At the beginning, 978 articles were extracted from three databases using the relevant keywords, and after excluding the duplicate items, 600 papers remained. 450 articles were excluded because of the subject irrelevance of titles and abstracts by three reviewers. 36 articles were excluded due to lacking full text, and being reviewed and letter. Finally, 114 articles remained and were used for this study (Diagram 1).
1. SARS-CoV-2
Coronavirus Disease 2019 (COVID-19) is a pandemic disease with severe pulmonary damage and hyper inflammation sign (44). Vitamin A considered as an important factor in the development of immune system responses and the regulation of acute inflammation. Vitamin A by the development of normal lung tissue and tissue repair after injury due to infection (45) may play a key role in the recovery after severe COVID-19 pneumonia (46). Vitamin A has the ability in immune regulatory functions by affecting the innate and adaptive immune cell response (47, 48). The deficiency in Vitamin A may disrupt vaccine-induced plasma cells and affect immunoglobulin development in the respiratory tracts (49, 50). Normally, Vitamin A (VA) has benefits in physiological functions, such as promoting growth and reproduction and maintaining bone, epithelial tissue, vision, and normal secretion of mucosal epithelium. Also, derivatives of VA can suppress precancerous lesions. This factor is necessary for the maintenance of immune cells count and it can be affected in immune cell differentiation and proliferation(51, 52). Vitamin A considered as the factor for minimization of COVID- 19 adverse effects on the angiotensin system and medication-related adverse effects by improving respiratory health and diminishing inflammation and fibrosis(53). Therefore, according to COVID-19 inflammatory response/cytokine storm, especially the involvement of the liver, lung, and kidney, which further increase the risk of death in patients, vitamin A can combat the life-threatening disease(54).
2. HSV
Today the prevalence of genital herpes simplex virus (HSV) infections through HSV-1 and HSV-2 are increasing in population and as a result the incidence of neonatal herpes with its undeniable problems resulting in neurologic disabilities or perhaps death (55). Usually, HSV-1 and HSV-2 infection in newborns happens through the birth process, while the neonate is exposed to HSV shedding in the cervical or vaginal excretions (55, 56). The earlier studies have reported reduction in HSV infectivity through treatment with RA. But, the consequence of several mechanisms leads to fewer virus proliferation or the production of noninfectious viruses. Both of these options could be caused by the effect of RA nuclear receptors on various elements such as the transcription factors or host or HSV genomes or even the effect on viral proteins which are not responsible for replication (57). Studies have reported much evidence that suppression of N-glycosylation in HSV-1 glycoproteins with tunicamycin or any drugs that prevent N-linked glycosylation results in the proliferation of noninfectious HSV particles (58, 59). These studies have shown that RA treatment of HSV-1 infected Vero cells is capable to decrease the proliferation of infectious particles through producing viral glycoproteins with lower molecular weights. Based on previous examinations it has been revealed that RA can cause accumulation of gB, gC and gD viral ligands in variable molecular weights suggesting that RA can interfere in protein glycosylation (60).
3. HCV
Several studies have been revealed that VAD is common in cirrhosis patients and its occurrence is higher in individuals with Hepatitis C virus (HCV) induced cirrhosis than other patients with the same sex, age and economic situation (61-63). In previous studies HCV positive cirrhosis Patients have been tested for vitamin A and these reports that the majority of inadequate vitamin A intake is common in them. Furthermore, in addition to VAD, they had higher serum bilirubin and serum albumin toward people with normal vitamin. VAD is related to Child-Pugh class (64). Outcomes of all studies are similar in high incidence of VAD in individuals with HCV-induced cirrhosis (65). Cirrhosis is linked ta o series biological happenings such as a hypermetabolic situation, more protein catabolism, induced lipid oxidation, reduced glycogen storage and glucose oxidation, and other factors of poor nutritional status (66). It has been shown that triggered lipid oxidation product including hydroxynonenal and malondialdehyde (67) can stimulate hepatic stellate cells (HSCs) in HCV-infected patients. In a healthy liver, HSCs are known as the main storage spots of vitamin A. HSCs in liver damaged conditions miss their retinol and excrete a noticeable measure of extracellular matrix with more collagen I, resulting in liver fibrosis (68, 69). In HSCs, retinyl esters carried in lipid particles hydrolyze and subsequently excrete from cells as retinol (70). The retinyl ester amount in the cells depends on the synthesis of lecithin retacyltransferaseerase, the single hepatic enzyme able to retinyl ester production in vivo(71). Other effectors related to decreasing of serum retinol measure in chronic liver disease have been revealed as a dietary lacking vitamin A supplement and decreased synthesis of retinol-binding protein in hepatocytes, because of liver disorder protein-energy starvation (63, 72). Besides, triggered expression of Cytochrome P4502E1 (CYP2E1) in hepatic disorders (73) has been revealed to play a key role in the reduction of hepatic retinoic acid stores through simplifying deprivation of retinoic acid into other metabolites (64).
4. Noroviruses
One of the common reasons of acute gastroenteritis is Norovirus. The main symptoms related to this infectious disease consist of vomiting, diarrhea, nausea, abdominal pain, and fever for 3 days (74). Norovirus infection is one of the most important public health problems because of the absence of effective treatment or vaccine for this infection (74, 75). According to the latest studies, Norovirus infection has no significate effect on gut microbiota so this is not one of issues related to viral pathogenesis (76) . Based on epidemiological studies using of sufficient vitamin A ultimate reduction in this viral infection and clinical symptoms (77). One of the metabolites of vitamin A dietary is Retinoic acid that can be useful in stimulation of innate immune response against viral infection. During researching it has been confirmed that sufficient vitamin A supplementation influenced mortality and morbidity rate and with reduction of this factors, it can be affect viral gastrointestinal diseases (78). In other word during in vitro and in vivo studies, it has been shown that vitamin A interferes with human Norovirus 1 replication and antiviral effect of vitamin A on Norovirus has been identified. As one of effects of vitamin A on immune response, it is recognized that IFN-b expression plays a key role in immune responses during vitamin A treatment (79). Therefore, besides vitamin A effect on antiviral immune responses, RIG-1 signaling was stimulated against human Norovirus (79-81).
5. HIV
Regarding to clinical studies used vitamin A as a therapeutic component in child survived from acute measles, it has been suggested that this method of treatment can be useful against human immunodeficiency virus (HIV) infection (82, 83). During Epidemiological studies, it has been shown that vitamin A deficiency between HIV-infected pregnant women is a common problem (84-86), as well as drug users and addicts populations (83). In poor countries like African countries that using antiretroviral and prophylactic therapies are expensive, treatment with micronutrients like vitamin A can be best strategy to improve survival rate and reducing vertical transmission of HIV (87, 88). According clinical trials about effectiveness of vitamin A therapy for HIV-infected children, it was observed that using vitamin A as a therapeutic component can reduces diarrhea morbidity (24, 89) and increases the host's immunity (90). Almost Four clinical trial have been done in different countries such as Tanzania (87, 91), South Africa (92), Malawi (93), and Zimbabwe (94) to figure out that whether vertical transmission and mortality rate of infant can be reduced by antenatal vitamin A supplementation (95). In other study periodic vitamin A supplementation have been investigated in one clinical trial in Uganda to study whether this periodic supplementation can be useful in improvement of survival of HIV infected children (96). By World Bank (1993) analysis between forty-seven major health interventions studies using vitamin A it has been shown that vitamin A has the second-highest cost-benefit effect in patients. There are some aspects of study on vitamin A's effects as a beneficial supplementation in reduction of vertical transmission in HIV infection (97), and infant mortality that remained unknown and needs more studies (84, 98, 99).
6. Entrovirus71
One of the common viral infections in under four years’ children is Hand, foot, and mouth disease (HFMD) related t3o Entroviruses. Skin and mucous membranes are major regions that get influenced by this viruses (100, 101). Based on the recent data of in-vitro studies (28, 102, 103) which revealed that retinoic acid receptor (RAR)-a is critical for the antiviral outcome of ATRA, current studies assessed the influences of variation of RAR-a expression through ATRA in EV71-infected cells (104). The quantitative PCR has discovered that ATRA may be efficient in up-regulation of RAR-a expression in either infected U937 (monocytes progenitor cell line) cells and non-infected cells (105). Nevertheless, by adding arotinoid ethyl ester (Ro) to the cell culture get understood that adding this factor prevents increasing the expression of RAR-α mRNA. So it can be concluded that, expression of RAR-a mRNA not affected by EV71 infection itself (106-108). RIG-I is a main IFN triggering gene that in response to EV71 can induce excretion of IFN-a (109, 110). For investigation of the antiviral effect of ATRA on IFN-a signaling, estimating the measure of RIG-I mRNA and genes in the downstream of signaling pathway carried out (100). Previous studies showed that in the lacking vitamin A treatment condition of U937 cells, RIG-I mRNA expression is in low levels. Also through ATRA treatment alone without other factors moderate increase in the expression of RIG-I mRNA was observed, but in EV71 infection alone, expression of RIG-I mRNA in this cell line not found. When the U937 cells get infected with EV71, for treatment of these cells ATRA was used as an effective factor to exhibited higher levels of RIG-I mRNA expression than cells that get treated with ATRA alone. In addition to modifications by effect of ATRA-treated and EV71-infected U937 cell model, it was understood that increase of expression of several downstream genes (IFN promoter-stimulating factor 1 (IPS-1), TRAF family member-associated NF-kB activator-binding kinase 1 (TBK1) TNF receptor-associated factor 3 (TRAF3) and interferon regulatory factor 3 (IRF3) in the RIG-I signaling pathway can be influence by this factor (104, 111). Thus, it can be mentioned that treatment with ATRA can activate RIG-I signaling in EV71-infected cells. Ro is the factor that can block the induction of RIG-I mRNA expression in EV71-infected cells that get treated with ATRA, so it means that up-regulation of the expression of this gene is mediated by RAR-a (104).
7. HPV
Cervical lesions are common pathological damages in women (112). One of the most important causes of these lesions is human papillomaviruses (HPV) infection, and during expression of viral transforming genes, neoplastic modification can be seen in these lesions (113). It has been revealed that DNA of HPV types 16 and 18 that are oncogenic HPVs can immortalize cultured human keratinocytes (HKc) and human cervical cells so this cell line can be a model to study molecular mechanisms of cervical carcinogenesis(114-119).
HKc infected with HPV16 primarily undergo to malignancy developments with a chain of identified phenotypic occurrences in vitro, such as growth factor independence and differentiation resistance,(117, 120) but HKc are consequently vulnerable to malignancy transformations after transfection with a viral or host cell Ras oncogene (121, 122). Based on the previous studies it can be concluded that HKc cells infected with HPV16 are more sensitive to growth and differentiation control by managing with all-trans-retinoic acid (RA), an active metabolite of vitamin A (123).
Additionally, when RA uses as a treatment for HKc cells infected with HPV16, it can reduce steadily the levels of the HPV16 oncogenes E6 and E7 mRNA and protein (124-126). Also physiologic levels of RA (1 nM) influenced immortalized situation of cells, as an inhibitor factor of HPV16-mediated immortalization of normal HKc approximately 95% (124, 125). Previous studies revealed that RA treatment can be an activator factor for triggering of the production of the growth suppressor transforming growth factor-β (TGF-β) (127, 128) in normal HKc and HKc/HPV16, suggested that RA suppression of growth is mediated via the TGF-β (129). This result is the same as findings of Wood worth et al. (1990) that considered, TGF- β as an inhibitor for E6 (130) and E7 (131) .
At the beginning, 978 articles were extracted from three databases using the relevant keywords, and after excluding the duplicate items, 600 papers remained. 450 articles were excluded because of the subject irrelevance of titles and abstracts by three reviewers. 36 articles were excluded due to lacking full text, and being reviewed and letter. Finally, 114 articles remained and were used for this study (Diagram 1).
1. SARS-CoV-2
Coronavirus Disease 2019 (COVID-19) is a pandemic disease with severe pulmonary damage and hyper inflammation sign (44). Vitamin A considered as an important factor in the development of immune system responses and the regulation of acute inflammation. Vitamin A by the development of normal lung tissue and tissue repair after injury due to infection (45) may play a key role in the recovery after severe COVID-19 pneumonia (46). Vitamin A has the ability in immune regulatory functions by affecting the innate and adaptive immune cell response (47, 48). The deficiency in Vitamin A may disrupt vaccine-induced plasma cells and affect immunoglobulin development in the respiratory tracts (49, 50). Normally, Vitamin A (VA) has benefits in physiological functions, such as promoting growth and reproduction and maintaining bone, epithelial tissue, vision, and normal secretion of mucosal epithelium. Also, derivatives of VA can suppress precancerous lesions. This factor is necessary for the maintenance of immune cells count and it can be affected in immune cell differentiation and proliferation(51, 52). Vitamin A considered as the factor for minimization of COVID- 19 adverse effects on the angiotensin system and medication-related adverse effects by improving respiratory health and diminishing inflammation and fibrosis(53). Therefore, according to COVID-19 inflammatory response/cytokine storm, especially the involvement of the liver, lung, and kidney, which further increase the risk of death in patients, vitamin A can combat the life-threatening disease(54).
2. HSV
Today the prevalence of genital herpes simplex virus (HSV) infections through HSV-1 and HSV-2 are increasing in population and as a result the incidence of neonatal herpes with its undeniable problems resulting in neurologic disabilities or perhaps death (55). Usually, HSV-1 and HSV-2 infection in newborns happens through the birth process, while the neonate is exposed to HSV shedding in the cervical or vaginal excretions (55, 56). The earlier studies have reported reduction in HSV infectivity through treatment with RA. But, the consequence of several mechanisms leads to fewer virus proliferation or the production of noninfectious viruses. Both of these options could be caused by the effect of RA nuclear receptors on various elements such as the transcription factors or host or HSV genomes or even the effect on viral proteins which are not responsible for replication (57). Studies have reported much evidence that suppression of N-glycosylation in HSV-1 glycoproteins with tunicamycin or any drugs that prevent N-linked glycosylation results in the proliferation of noninfectious HSV particles (58, 59). These studies have shown that RA treatment of HSV-1 infected Vero cells is capable to decrease the proliferation of infectious particles through producing viral glycoproteins with lower molecular weights. Based on previous examinations it has been revealed that RA can cause accumulation of gB, gC and gD viral ligands in variable molecular weights suggesting that RA can interfere in protein glycosylation (60).
3. HCV
Several studies have been revealed that VAD is common in cirrhosis patients and its occurrence is higher in individuals with Hepatitis C virus (HCV) induced cirrhosis than other patients with the same sex, age and economic situation (61-63). In previous studies HCV positive cirrhosis Patients have been tested for vitamin A and these reports that the majority of inadequate vitamin A intake is common in them. Furthermore, in addition to VAD, they had higher serum bilirubin and serum albumin toward people with normal vitamin. VAD is related to Child-Pugh class (64). Outcomes of all studies are similar in high incidence of VAD in individuals with HCV-induced cirrhosis (65). Cirrhosis is linked ta o series biological happenings such as a hypermetabolic situation, more protein catabolism, induced lipid oxidation, reduced glycogen storage and glucose oxidation, and other factors of poor nutritional status (66). It has been shown that triggered lipid oxidation product including hydroxynonenal and malondialdehyde (67) can stimulate hepatic stellate cells (HSCs) in HCV-infected patients. In a healthy liver, HSCs are known as the main storage spots of vitamin A. HSCs in liver damaged conditions miss their retinol and excrete a noticeable measure of extracellular matrix with more collagen I, resulting in liver fibrosis (68, 69). In HSCs, retinyl esters carried in lipid particles hydrolyze and subsequently excrete from cells as retinol (70). The retinyl ester amount in the cells depends on the synthesis of lecithin retacyltransferaseerase, the single hepatic enzyme able to retinyl ester production in vivo(71). Other effectors related to decreasing of serum retinol measure in chronic liver disease have been revealed as a dietary lacking vitamin A supplement and decreased synthesis of retinol-binding protein in hepatocytes, because of liver disorder protein-energy starvation (63, 72). Besides, triggered expression of Cytochrome P4502E1 (CYP2E1) in hepatic disorders (73) has been revealed to play a key role in the reduction of hepatic retinoic acid stores through simplifying deprivation of retinoic acid into other metabolites (64).
4. Noroviruses
One of the common reasons of acute gastroenteritis is Norovirus. The main symptoms related to this infectious disease consist of vomiting, diarrhea, nausea, abdominal pain, and fever for 3 days (74). Norovirus infection is one of the most important public health problems because of the absence of effective treatment or vaccine for this infection (74, 75). According to the latest studies, Norovirus infection has no significate effect on gut microbiota so this is not one of issues related to viral pathogenesis (76) . Based on epidemiological studies using of sufficient vitamin A ultimate reduction in this viral infection and clinical symptoms (77). One of the metabolites of vitamin A dietary is Retinoic acid that can be useful in stimulation of innate immune response against viral infection. During researching it has been confirmed that sufficient vitamin A supplementation influenced mortality and morbidity rate and with reduction of this factors, it can be affect viral gastrointestinal diseases (78). In other word during in vitro and in vivo studies, it has been shown that vitamin A interferes with human Norovirus 1 replication and antiviral effect of vitamin A on Norovirus has been identified. As one of effects of vitamin A on immune response, it is recognized that IFN-b expression plays a key role in immune responses during vitamin A treatment (79). Therefore, besides vitamin A effect on antiviral immune responses, RIG-1 signaling was stimulated against human Norovirus (79-81).
5. HIV
Regarding to clinical studies used vitamin A as a therapeutic component in child survived from acute measles, it has been suggested that this method of treatment can be useful against human immunodeficiency virus (HIV) infection (82, 83). During Epidemiological studies, it has been shown that vitamin A deficiency between HIV-infected pregnant women is a common problem (84-86), as well as drug users and addicts populations (83). In poor countries like African countries that using antiretroviral and prophylactic therapies are expensive, treatment with micronutrients like vitamin A can be best strategy to improve survival rate and reducing vertical transmission of HIV (87, 88). According clinical trials about effectiveness of vitamin A therapy for HIV-infected children, it was observed that using vitamin A as a therapeutic component can reduces diarrhea morbidity (24, 89) and increases the host's immunity (90). Almost Four clinical trial have been done in different countries such as Tanzania (87, 91), South Africa (92), Malawi (93), and Zimbabwe (94) to figure out that whether vertical transmission and mortality rate of infant can be reduced by antenatal vitamin A supplementation (95). In other study periodic vitamin A supplementation have been investigated in one clinical trial in Uganda to study whether this periodic supplementation can be useful in improvement of survival of HIV infected children (96). By World Bank (1993) analysis between forty-seven major health interventions studies using vitamin A it has been shown that vitamin A has the second-highest cost-benefit effect in patients. There are some aspects of study on vitamin A's effects as a beneficial supplementation in reduction of vertical transmission in HIV infection (97), and infant mortality that remained unknown and needs more studies (84, 98, 99).
6. Entrovirus71
One of the common viral infections in under four years’ children is Hand, foot, and mouth disease (HFMD) related t3o Entroviruses. Skin and mucous membranes are major regions that get influenced by this viruses (100, 101). Based on the recent data of in-vitro studies (28, 102, 103) which revealed that retinoic acid receptor (RAR)-a is critical for the antiviral outcome of ATRA, current studies assessed the influences of variation of RAR-a expression through ATRA in EV71-infected cells (104). The quantitative PCR has discovered that ATRA may be efficient in up-regulation of RAR-a expression in either infected U937 (monocytes progenitor cell line) cells and non-infected cells (105). Nevertheless, by adding arotinoid ethyl ester (Ro) to the cell culture get understood that adding this factor prevents increasing the expression of RAR-α mRNA. So it can be concluded that, expression of RAR-a mRNA not affected by EV71 infection itself (106-108). RIG-I is a main IFN triggering gene that in response to EV71 can induce excretion of IFN-a (109, 110). For investigation of the antiviral effect of ATRA on IFN-a signaling, estimating the measure of RIG-I mRNA and genes in the downstream of signaling pathway carried out (100). Previous studies showed that in the lacking vitamin A treatment condition of U937 cells, RIG-I mRNA expression is in low levels. Also through ATRA treatment alone without other factors moderate increase in the expression of RIG-I mRNA was observed, but in EV71 infection alone, expression of RIG-I mRNA in this cell line not found. When the U937 cells get infected with EV71, for treatment of these cells ATRA was used as an effective factor to exhibited higher levels of RIG-I mRNA expression than cells that get treated with ATRA alone. In addition to modifications by effect of ATRA-treated and EV71-infected U937 cell model, it was understood that increase of expression of several downstream genes (IFN promoter-stimulating factor 1 (IPS-1), TRAF family member-associated NF-kB activator-binding kinase 1 (TBK1) TNF receptor-associated factor 3 (TRAF3) and interferon regulatory factor 3 (IRF3) in the RIG-I signaling pathway can be influence by this factor (104, 111). Thus, it can be mentioned that treatment with ATRA can activate RIG-I signaling in EV71-infected cells. Ro is the factor that can block the induction of RIG-I mRNA expression in EV71-infected cells that get treated with ATRA, so it means that up-regulation of the expression of this gene is mediated by RAR-a (104).
7. HPV
Cervical lesions are common pathological damages in women (112). One of the most important causes of these lesions is human papillomaviruses (HPV) infection, and during expression of viral transforming genes, neoplastic modification can be seen in these lesions (113). It has been revealed that DNA of HPV types 16 and 18 that are oncogenic HPVs can immortalize cultured human keratinocytes (HKc) and human cervical cells so this cell line can be a model to study molecular mechanisms of cervical carcinogenesis(114-119).
HKc infected with HPV16 primarily undergo to malignancy developments with a chain of identified phenotypic occurrences in vitro, such as growth factor independence and differentiation resistance,(117, 120) but HKc are consequently vulnerable to malignancy transformations after transfection with a viral or host cell Ras oncogene (121, 122). Based on the previous studies it can be concluded that HKc cells infected with HPV16 are more sensitive to growth and differentiation control by managing with all-trans-retinoic acid (RA), an active metabolite of vitamin A (123).
Additionally, when RA uses as a treatment for HKc cells infected with HPV16, it can reduce steadily the levels of the HPV16 oncogenes E6 and E7 mRNA and protein (124-126). Also physiologic levels of RA (1 nM) influenced immortalized situation of cells, as an inhibitor factor of HPV16-mediated immortalization of normal HKc approximately 95% (124, 125). Previous studies revealed that RA treatment can be an activator factor for triggering of the production of the growth suppressor transforming growth factor-β (TGF-β) (127, 128) in normal HKc and HKc/HPV16, suggested that RA suppression of growth is mediated via the TGF-β (129). This result is the same as findings of Wood worth et al. (1990) that considered, TGF- β as an inhibitor for E6 (130) and E7 (131) .

8. Mumps
U937 cells are considered as neoplastic and histiocytic progenitors of monocytes that in many times during immunological studies have been used (132). These cells specially are an important case during Mumps virus (MuV) infection to studying of interferon pathways(133-131). During studies get understood that increasing doses of retinol in these cells act as an inhibitor for MuV replication and the quantity of process measured by TCID50. It was observed that concentrations as low as 1 μM, act as suitable inhibitor and also with this dose increased expression of the retinoid responsive gene RARβ was observed. Increasing doses of ATRA during treatment of U937 cells have efficient operation as inhibitor of MuV output and are effective in the induction of RARβ mRNA expression (132). Several studies reported ATRA at a dose of 1 μM have antiviral effect on MuV. Also IFN signaling increases during the Retinoid treatment. As a primary control of infection, the innate immune response thought to be responsible for immunity and it is mentioned that up-regulation of the type I IFN response functions have an important role in antiviral responses. When MuV infection alone occurred in the U937 model, it causes induction in expression of IFNα1 mRNA. Also, using ATRA as a treatment of MuV infected cells act as a cofactor and its operation has a synergism action to increase the expression of IFNα1 mRNA and its protein levels. So with these concepts it can be assumed that expression of IFNβ mRNA and its protein levels, during treatment of ATRA in MuV infection will get increased. The expression of ISGs will be influenced by increasing in type I IFN production. Also, over the treatment by ATRA in the U937 model, (106) IRF-1 mRNA expression get increased, and this result is favorable in some previous studies and literatures (108, 133, 134). So for expression of RIG-I mRNA, treatment with ATRA is required (Table1) (132).
Conclusion
The promising role of vitamin A in different viral infections is well‐described by several epidemiological studies, supporting the notion that higher level of vitamin A is associated with better prognosis and improved outcomes. Although the mechanisms responsible for vitamin A function in the host immune system have been widely described, the interplay between viral infections and vitamin A status remains an intriguing area, and the potential interactions between viral infections and vitamin A appears to be more complex than our previous knowledge. Induction of antimicrobial peptides, immunoregulatory function, interaction with cellular and viral factors are the main underlying mechanisms by which vitamin A insufficiency could contribute to viral disease development. These Data demonstrate that retinoid can inhibit viral replication commonly through a retinoid inducible gene I (RIG-I), Retinoic Acid Receptor (RAR) and IFN dependent manner making them refractory to subsequent rounds of viral replication. These observations raise the possibility that pharmacological doses of retinoids might have clinical benefit in different viral infections.
Declaration
This study has been conducted in Microbiology Department of the School of Medicine Golestan University of Medical Sciences.
Conflict of interest
The authors declare no conflicts of interests.
Type of Article: Review Article |
Subject:
Basic Medical Sciences
Received: 2021/12/14 | Accepted: 2022/03/6 | Published: 2022/03/30
Received: 2021/12/14 | Accepted: 2022/03/6 | Published: 2022/03/30
References
1. D'Ambrosio D, Clugston R, Blaner W, D'Ambrosio D, Clugston R, Blaner W. Vitamin A Metabolism: An Update. Nutrients, 3, 63-103. 2011. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
2. Napoli JL. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clinical immunology and immunopathology. 1996;80(3):S52-S62. [view at publisher] [DOI] [PMID] [Google Scholar]
3. Joint F. WHO Expert Consultation on Human vitamin and mineral requirements. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand. 1998;2. [Google Scholar]
4. Warkany J, Schraffenberger E. Congenital malformations induced in rats by maternal vitamin A deficiency: I. Defects of the eye. Archives of ophthalmology. 1946;35(2):150-69. [view at publisher] [DOI] [PMID] [Google Scholar]
5. Patel S, Vajdy M. Induction of cellular and molecular immunomodulatory pathways by vitamin A and flavonoids. Expert opinion on biological therapy. 2015;15(10):1411-28. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
6. Yu M, Vajdy M. A novel retinoic acid, catechin hydrate and mustard oil-based emulsion for enhanced cytokine and antibody responses against multiple strains of HIV-1 following mucosal and systemic vaccinations. Vaccine. 2011;29(13):2429-36. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
7. Vajdy M. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert opinion on biological therapy. 2011;11(11):1501-13. [view at publisher] [DOI] [PMID] [Google Scholar]
8. D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3(1):63-103. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
9. Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiological reviews. 1991;71(4):951-90. [view at publisher] [DOI] [PMID] [Google Scholar]
10. Ong DE. Absorption of vitamin A. Vitamin A in health and disease: Marcel Dekker, New York; 1994. p. 37-72.
11. Parker RS. Absorption, metabolism, and transport of carotenoids. The FASEB Journal. 1996;10(5):542-51. [DOI] [PMID]
12. Stephensen CB. Vitamin A, infection, and immune function. Annual review of nutrition. 2001;21(1):167-92. [view at publisher] [DOI] [PMID] [Google Scholar]
13. Engedal N, Gjevik T, Blomhoff R, Blomhoff HK. All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: early induction of cyclin D3. The Journal of Immunology. 2006;177(5):2851-61. [view at publisher] [DOI] [PMID] [Google Scholar]
14. Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451-80. [view at publisher] [DOI] [PMID] [Google Scholar]
15. Barclay A, Foster A, Sommer A. Vitamin A supplements and mortality related to measles: a randomised clinical trial. Br Med J (Clin Res Ed). 1987;294(6567):294-6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
16. Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized, placebo-controlled, double-blind trial. The American journal of clinical nutrition. 1991;54(5):890-5. [view at publisher] [DOI] [PMID] [Google Scholar]
17. Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. The American journal of clinical nutrition. 1984;40(5):1090-5. [view at publisher] [DOI] [PMID] [Google Scholar]
18. Hossain S, Biswas R, Kabir I, Sarker S, Dibley M, Fuchs G, et al. Single dose vitamin A treatment in acute shigellosis in Bangladeshi children: randomised double blind controlled trial. Bmj. 1998;316(7129):422-6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
19. Neuman MI, Willett WC, Curhan GC. Vitamin and micronutrient intake and the risk of community-acquired pneumonia in US women. The American journal of medicine. 2007;120(4):330-6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
20. Rahman MM, Mahalanabis D, Alvarez J, Wahed M, Islam M, Habte D, et al. Acute respiratory infections prevent improvement of vitamin A status in young infants supplemented with vitamin A. The Journal of nutrition. 1996;126(3):628-33. [view at publisher] [DOI] [PMID] [Google Scholar]
21. Metzger A, Mukasa G, Shankar AH, Ndeezi G, Melikian G, Semba RD. Antioxidant status and acute malaria in children in Kampala, Uganda. The American journal of tropical medicine and hygiene. 2001;65(2):115-9. [DOI] [PMID] [Google Scholar]
22. Nussenblatt V, Semba RD. Micronutrient malnutrition and the pathogenesis of malarial anemia. Acta Tropica. 2002;82(3):321-37. [view at publisher] [DOI] [Google Scholar]
23. Semba RD, Tang AM. Micronutrients and the pathogenesis of human immunodeficiency virus infection. British Journal of Nutrition. 1999;81(3):181-9. [view at publisher] [DOI] [PMID] [Google Scholar]
24. Coutsoudis A, Bobat R, Coovadia HM, Kuhn L, Tsai W-Y, Stein ZA. The effects of vitamin A supplementation on the morbidity of children born to HIV-infected women. American Journal of Public Health. 1995;85(8_Pt_1):1076-81. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
25. Penkert RR, Jones BG, Häcker H, Partridge JF, Hurwitz JL. Vitamin A differentially regulates cytokine expression in respiratory epithelial and macrophage cell lines. Cytokine. 2017;91:1-5. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
26. Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proceedings of the Nutrition Society. 1999;58(3):719-27. [view at publisher] [DOI] [PMID] [Google Scholar]
27. Stošić-Grujičić S, Ejdus L. Modulation of in vitro T cell alloreactivity by synthetic retinoids. Immunopharmacology. 1994;27(2):87-92. [view at publisher] [DOI] [Google Scholar]
28. Dong P, Tao Y, Yang Y, Wang W. Expression of retinoic acid receptors in intestinal mucosa and the effect of vitamin A on mucosal immunity. Nutrition. 2010;26(7-8):740-5. [view at publisher] [DOI] [PMID] [Google Scholar]
29. Kaufman DR, De Calisto J, Simmons NL, Cruz AN, Villablanca EJ, Mora JR, et al. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. The Journal of Immunology. 2011;187(4):1877-83. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
30. Semba RD, Scott AL, Natadisastra G, Wirasasmita S, Mele L, Ridwan E, et al. Depressed immune response to tetanus in children with vitamin A deficiency. The Journal of nutrition. 1992;122(1):101-7. [view at publisher] [DOI] [PMID] [Google Scholar]
31. Semba RD, Scott AL, Natadisastra G, West K, Sommer A. Effect of vitamin A supplementation on immunoglobulin G subclass responses to tetanus toxoid in children. Clin Diagn Lab Immunol. 1994;1(2):172-5. [DOI] [PMID] [PMCID] [Google Scholar]
32. Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157-60. [view at publisher] [DOI] [PMID] [Google Scholar]
33. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527-38. [view at publisher] [DOI] [PMID] [Google Scholar]
34. Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424(6944):88-93. [view at publisher] [DOI] [PMID] [Google Scholar]
35. Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin-and gut-associated lymphoid tissues. The Journal of experimental medicine. 2005;201(2):303-16. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
36. McDonald SL, Savy M, Fulford AJ, Kendall L, Flanagan KL, Prentice AM. A double blind randomized controlled trial in neonates to determine the effect of vitamin A supplementation on immune responses: The Gambia protocol. BMC pediatrics. 2014;14(1):92. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
37. Carman J, Hayes C. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. The Journal of Immunology. 1991;147(4):1247-52. [view at publisher] [Google Scholar]
38. Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. The Journal of Immunology. 1994;152(4):1515-22. [view at publisher] [Google Scholar]
39. Jones LH, Cook PC, Ivens AC, Thomas G, D, Phythian-Adams AT, Allen JE, et al. Modulation of dendritic cell alternative activation and function by the vitamin A metabolite retinoic acid. International immunology. 2015;27(11):589-96. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
40. Surman SL, Jones BG, Rudraraju R, Sealy RE, Hurwitz JL. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin Vaccine Immunol. 2014;21(4):598-601. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
41. Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One. 2014;9(1). [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
42. Semba RD. The role of vitamin A and related retinoids in immune function. Nutrition reviews. 1998;56(1):S38-S48. [DOI] [PMID] [Google Scholar]
43. Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. The Journal of Immunology. 2008;181(4):2277-84. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
44. Behboudi E, Hamidi V, Gholizadeh F, Grala EM, Ghelmani Y, Nakhaie M, et al. Association between ABO blood groups and rhesus antigen and susceptibility to COVID-19 in the Yazd hospital. New Microbes and New infections. 2021;44:100934. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
45. Timoneda J, Rodríguez-Fernández L, Zaragozá R, Marín MP, Cabezuelo MT, Torres L, et al. Vitamin A Deficiency and the Lung. Nutrients. 2018;10(9). [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
46. Chew BP, Park JS. Carotenoid action on the immune response. The Journal of nutrition. 2004;134(1):257s-61s. [view at publisher] [DOI] [PMID] [Google Scholar]
47. Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. Journal of immunology (Baltimore, Md : 1950). 2014;192(7):2953-8. [view at publisher] [DOI] [PMID] [Google Scholar]
48. Behboudi E, Shamsi A, Hamidi-Sofiani V, Oladnabi MJIJoP. The effects of fasting in Ramadan on the risk factors of COVID-19 in adolescents: a brief review. International Journal of Pediatrics. 2021;9(1):12835-42. [Google Scholar]
49. Surman SL, Rudraraju R, Sealy R, Jones B, Hurwitz JL. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 2012;25(4):341-4. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
50. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of Vitamin A in the Immune System. J Clin Med. 2018;7(9):258. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
51. Behboudi E, Hamidi-Sofiani V, Zeynali P. Review of Therapeutic Candidates for the New Corona Virus Disease (COVID-19). Razi Journal of Medical Sciences. 2020;27(8):65-77. [view at publisher] [Google Scholar]
52. Sarohan AR. COVID-19: Endogenous Retinoic Acid Theory and Retinoic Acid Depletion Syndrome. Med Hypotheses. 2020;144:110250-. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
53. Thirumdas R, Kothakota A, Pandiselvam R, Bahrami A, Barba FJ. Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: A review. Trends in food science & technology. 2021;110:66-77. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
54. Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, et al. The acquisition of herpes simplex virus during pregnancy. New England Journal of Medicine. 1997;337(8):509-16. [view at publisher] [DOI] [PMID] [Google Scholar]
55. Whitley RJ. Herpes simplex virus infections of women and their offspring: implications for a developed society. Proceedings of the National Academy of Sciences. 1994;91(7):2441-7. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
56. Katz E, Margalith E, Duksin D. Antiviral activity of tunicamycin on herpes simplex virus. Antimicrobial agents and chemotherapy. 1980;17(6):1014-22. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
57. Kousoulas KG, Bzik DJ, Deluca N, Person S. The effect of ammonium chloride and tunicamycin on the glycoprotein content and infectivity of herpes simplex virus type 1. Virology. 1983;125(2):468-74. [view at publisher] [DOI] [Google Scholar]
58. Isaacs CE, Xu W, Pullarkat RK, Kascsak R. Retinoic acid reduces the yield of herpes simplex virus in Vero cells and alters the N-glycosylation of viral envelope proteins. Antiviral research. 2000;47(1):29-40. [view at publisher] [DOI] [Google Scholar]
59. Guglielmi F, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism: multicentre prospective study by the 'Nutritional Problems in Gastroenterology'Section of the Italian Society of Gastroenterology (SIGE). Digestive and liver disease. 2005;37(9):681-8. [view at publisher] [DOI] [PMID] [Google Scholar]
60. Russell R, Iber F, Krasinski S, Miller P. Protein-energy malnutrition and liver dysfunction limit the usefulness of the relative dose response (RDR) test for predicting vitamin A deficiency. Human nutrition Clinical nutrition. 1983;37(5):361-71. [view at publisher] [Google Scholar]
61. Ukleja A, Scolapio JS, McConnell JP, Spivey JR, Dickson RC, Nguyen JH, et al. Nutritional assessment of serum and hepatic vitamin A levels in patients with cirrhosis. Journal of Parenteral and Enteral Nutrition. 2002;26(3):184-8. [view at publisher] [DOI] [PMID] [Google Scholar]
62. El-Eshmawy MM, Arafa MM, Elzehery RR, Elhelaly RM, Elrakhawy MM, El-Baiomy AA. Relationship between vitamin A deficiency and the thyroid axis in clinically stable patients with liver cirrhosis related to hepatitis C virus. Applied Physiology, Nutrition, and Metabolism. 2016;41(9):985-91. [DOI] [PMID] [Google Scholar]
63. Peres W, Chaves G, Gonçalves J, Ramalho A, Coelho H. Vitamin A deficiency in patients with hepatitis C virus-related chronic liver disease. British journal of nutrition. 2011;106(11):1724-31. [DOI] [PMID]
64. Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. Journal of gastroenterology and hepatology. 2008;23(4):527-33. [view at publisher] [DOI] [PMID] [Google Scholar]
65. De Maria N, Colantonl A, Fagiuoli S, Liu G-J, Rogers BK, Farinati F, et al. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radical Biology and Medicine. 1996;21(3):291-5. [view at publisher] [DOI] [Google Scholar]
66. Bataller R, David A. Brenner Da. Liver fibrosis J Clin Invest. 2005;115:209-18. [DOI] [PMID] [PMCID] [Google Scholar]
67. Griswold MD. Spermatogenesis: the commitment to meiosis. Physiological reviews. 2016;96(1):1-17. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
68. Friedman SL, Wei S, Blaner WS. Retinol release by activated rat hepatic lipocytes: regulation by Kupffer cell-conditioned medium and PDGF. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1993;264(5):G947-G52. [view at publisher] [DOI] [PMID] [Google Scholar]
69. O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, et al. Retinoid absorption and storage is impaired in mice lacking lecithin: retinol acyltransferase (LRAT). Journal of Biological Chemistry. 2005;280(42):35647-57. [DOI] [PMID] [PMCID] [Google Scholar]
70. Calamita Z, Dichi I, Papini-Berto SJ, Dichi JB, Angeleli A, Vannucchi H, et al. Plasma levels of transthyretin and retinol-binding protein in Child-A cirrhotic patients in relation to protein-calorie status and plasma amino acids, zinc, vitamin A and plasma thyroid hormones. Arquivos de gastroenterologia. 1997;34(3):139-47. [view at publisher] [Google Scholar]
71. Aubert J, Begriche K, Knockaert L, Robin M-A, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clinics and research in hepatology and gastroenterology. 2011;35(10):630-7. [view at publisher] [DOI] [PMID] [Google Scholar]
72. AhmedSM L. LevyK. Asystematicreview andmetaranalysisoftheglobalseasonalityofnorovirus. 2013;8(10):e75922. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
73. Hall AJ, Eisenbart VG, Etingüe AL, Gould LH, Lopman BA, Parashar UD. Epidemiology of foodborne norovirus outbreaks, United States, 2001-2008. Emerging infectious diseases. 2012;18(10):1566. [DOI] [PMID] [PMCID] [Google Scholar]
74. Nelson AM, Elftman MD, Pinto AK, Baldridge M, Hooper P, Kuczynski J, et al. Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome. 2013;1(1):7. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
75. Long KZ, García C, Santos JI, Rosado JL, Hertzmark E, DuPont HL, et al. Vitamin A supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. The Journal of infectious diseases. 2007;196(7):978-85. [view at publisher] [DOI] [PMID] [Google Scholar]
76. Thornton KA, Mora-Plazas M, Marín C, Villamor E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. The Journal of nutrition. 2014;144(4):496-503. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
77. Takeuchi O, Akira S. Innate immunity to virus infection. Immunological reviews. 2009;227(1):75-86. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
78. Lee H, Ko G. New perspectives regarding the antiviral effect of vitamin A on norovirus using modulation of gut microbiota. Gut Microbes. 2017;8(6):616-20. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
79. Lee H, Ko G. Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Scientific reports. 2016;6(1):1-9. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
80. Beaton GH. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. United Nations Subcommittee on Nutrition (SCN) News. 1993;9:17-23. [view at publisher] [Google Scholar]
81. Semba RD, Caiaffa WT, Graham NM, Cohn S, Vlahov D. Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users. Journal of Infectious Diseases. 1995;171(5):1196-202. [view at publisher] [DOI] [PMID] [Google Scholar]
82. Semba RD, Miotti PG, Chiphangwi JD, Liomba G, Yang L-P, Saah AJ, et al. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clinical Infectious Diseases. 1995;21(4):966-72. [view at publisher] [DOI] [PMID] [Google Scholar]
83. Burns DN, FitzGerald G, Semba R, Hershow R, Zorrilla C, Pitt J, et al. Vitamin A deficiency and other nutritional indices during pregnancy in human immunodeficiency virus infection: prevalence, clinical correlates, and outcome. Clinical Infectious Diseases. 1999;29(2):328-34. [view at publisher] [DOI] [PMID] [Google Scholar]
84. Semba RD, Miotti P, Chiphangwi JD, Henderson R, Dallabetta G, Yang L-P, et al. Maternal vitamin A deficiency and child growth failure during human immunodeficiency virus infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1997;14(3):219-22. [view at publisher] [DOI] [PMID] [Google Scholar]
85. Fawzi WW, Msamanga G, Hunter D, Urassa E, Renjifo B, Mwakagile D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV-1 in Tanzania. Journal of acquired immune deficiency syndromes (1999). 2000;23(3):246-54. [view at publisher] [DOI] [PMID] [Google Scholar]
86. Wiysonge CS, Ndze VN, Kongnyuy EJ, Shey MS. Vitamin A supplements for reducing mother‐to‐child HIV transmission. Cochrane Database of Systematic Reviews. 2017(9). [DOI] [PMID] [PMCID] [Google Scholar]
87. Filteau SM, Rollins NC, Coutsoudis A, Sullivan KR, Willumsen JF, Tomkins AM. The effect of antenatal vitamin A and β-carotene supplementation on gut integrity of infants of HIV-infected South African women. Journal of pediatric gastroenterology and nutrition. 2001;32(4):464-70. [view at publisher] [DOI] [PMID] [Google Scholar]
88. Hussey G, Hughes J, Potgieter S, Kessow G, Burgess J, Beatty D, et al., editors. Vitamin A status and supplementation and its effects on immunity in children with AIDS. Report of XVII IVACG Meeting; 1996. [Google Scholar]
89. Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. The Lancet. 1998;351(9114):1477-82. [view at publisher] [DOI] [Google Scholar]
90. Coutsoudis A, Pillay K, Spooner E, Kuhn L. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. Aids. 1999;13(12):1517-24. [view at publisher] [DOI] [PMID] [Google Scholar]
91. Kumwenda N, Miotti PG, Taha TE, Broadhead R, Biggar RJ, Jackson JB, et al. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clinical Infectious Diseases. 2002;35(5):618-24. [view at publisher] [DOI] [PMID] [Google Scholar]
92. Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. The American journal of clinical nutrition. 2005;81(2):454-60. [view at publisher] [DOI] [PMID] [Google Scholar]
93. Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Archives of internal medicine. 1993;153(18):2149-54. [view at publisher] [DOI] [PMID] [Google Scholar]
94. Semba RD, Ndugwa C, Perry RT, Clark TD, Jackson JB, Melikian G, et al. Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus-infected children in Uganda: a controlled clinical trial. Nutrition. 2005;21(1):25-31. [view at publisher] [DOI] [PMID] [Google Scholar]
95. Semba RD, Chiphangwi J, Miotti P, Dallabetta G, Hoover D, Canner J, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. The Lancet. 1994;343(8913):1593-7. [view at publisher] [DOI] [Google Scholar]
96. Semba RD. Vitamin A and human immunodeficiency virus infection. Proceedings of the Nutrition Society. 1997;56(1B):459-69. [view at publisher] [DOI] [PMID] [Google Scholar]
97. John GC, Nduati RW, Mbori-Ngacha D, Overbaugh J, Welch M, Richardson BA, et al. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. Journal of Infectious Diseases. 1997;175(1):57-62. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
98. Chen S, Yang Y, Yan X, Chen J, Yu H, Wang W. Influence of vitamin A status on the antiviral immunity of children with hand, foot and mouth disease. Clinical nutrition. 2012;31(4):543-8. [view at publisher] [DOI] [PMID] [Google Scholar]
99. Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, et al. Enterovirus 71 outbreak in the People's Republic of China in 2008. Journal of clinical microbiology. 2009;47(7):2351-2. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
100. Wei D, Yang Y, Wang W. The expression of retinoic acid receptors in lymph nodes of young children and the effect of all-trans-retinoic acid on the B cells from lymph nodes. Journal of clinical immunology. 2007;27(1):88. [view at publisher] [DOI] [PMID] [Google Scholar]
101. Zhou X, Wang W, Yang Y. The expression of retinoic acid receptors in thymus of young children and the effect of all-transretinoic acid on the development of T cells in thymus. Journal of clinical immunology. 2008;28(1):85-91. [view at publisher] [DOI] [PMID] [Google Scholar]
102. Chen S, Yang Y, Xu J, Su L, Wang W. Effect of all-trans-retinoic acid on enterovirus 71 infection in vitro. British Journal of Nutrition. 2014;111(9):1586-93. [view at publisher] [DOI] [PMID] [Google Scholar]
103. Wang S-M, Chen I-C, Su L-Y, Huang K-J, Lei H-Y, Liu C-C. Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin Vaccine Immunol. 2010;17(10):1517-23. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
104. Soye KJ, Trottier C, Richardson CD, Ward BJ, Miller Jr WH. RIG-I is required for the inhibition of measles virus by retinoids. PloS one. 2011;6(7). [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
105. Luo XM, Ross AC. Physiological and receptor-selective retinoids modulate interferon γ signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. Journal of Biological Chemistry. 2005;280(43):36228-36. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
106. Dimberg A, Nilsson K, Öberg F. Phosphorylation-deficient Stat1 inhibits retinoic acid-induced differentiation and cell cycle arrest in U-937 monoblasts. Blood, The Journal of the American Society of Hematology. 2000;96(8):2870-8. [view at publisher] [DOI] [Google Scholar]
107. Lu J, Yi L, Zhao J, Yu J, Chen Y, Lin MC, et al. Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1. Journal of virology. 2012;86(7):3767-76. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
108. Huang H-I, Weng K-F, Shih S-R. Viral and host factors that contribute to pathogenicity of enterovirus 71. Future microbiology. 2012;7(4):467-79. [view at publisher] [DOI] [PMID] [Google Scholar]
109. Gianni M, Terao M, Fortino I, LiCalzi M, Viggiano V, Barbui T, et al. Stat1 is induced and activated by all-trans retinoic acid in acute promyelocytic leukemia cells. Blood, The Journal of the American Society of Hematology. 1997;89(3):1001-12. [view at publisher] [DOI] [Google Scholar]
110. Pfister H. The role of human papillomavirus in anogenital cancer. Obstetrics and gynecology clinics of North America. 1996;23(3):579-95. [view at publisher] [Google Scholar]
111. Dürst M, Dzarlieva-Petrusevska R, Boukamp P, Fusenig N, Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251-6. [view at publisher] [Google Scholar]
112. Kaur P, McDOUGALL JK. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. Journal of virology. 1988;62(6):1917-24. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
113. Pirisi L, Yasumoto S, Feller M, Doniger J, DiPAOLO JA. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. Journal of virology. 1987;61(4):1061-6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
114. Pirisi L, Creek KE, Doniger J, Dipaolo JA. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988;9(9):1573-9. [view at publisher] [DOI] [PMID] [Google Scholar]
115. Woodworth C, Bowden PE, Doniger J, Pirisi L, Barnes W, Lancaster W, et al. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer research. 1988;48(16):4620-8. [view at publisher] [Google Scholar]
116. Woodworth C, Doniger J, DiPaolo J. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. Journal of Virology. 1989;63(1):159-64. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
117. Zyzak LL, MacDonald LM, Batova A, Forand R, Creek KE, Pirisi L. Increased levels and constitutive tyrosine phosphorylation of the epidermal growth factor receptor contribute to autonomous growth of human papillomavirus type 16 immortalized human keratinocytes. Cell Growth and Differentiation-Publication American Association for Cancer Research. 1994;5(5):537-48. [view at publisher] [Google Scholar]
118. DiPaolo J, Woodworth C, Popescu N, Notario V, Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989;4(4):395-9. [view at publisher] [Google Scholar]
119. Dipaolo JA, Woodworth CD, Popescu NC, Koval DL, Lopez JV, Doniger J. HSV-2-induced tumorigenicity in HPV16-immortalized human genital keratinocytes. Virology. 1990;177(2):777-9. [view at publisher] [DOI] [Google Scholar]
120. Pirisi L, Batova A, Jenkins GR, Hodam JR, Creek KE. Increased sensitivity of human keratinocytes immortalized by human papillomavirus type 16 DNA to growth control by retinoids. Cancer research. 1992;52(1):187-93. [view at publisher] [Google Scholar]
121. Khan MA, Jenkins GR, Tolleson WH, Creek KE, Pirisi L. Retinoic acid inhibition of human papillomavirus type 16-mediated transformation of human keratinocytes. Cancer research. 1993;53(4):905-9. [view at publisher] [Google Scholar]
122. Creek KE, Jenkins GR, Khan MA, Batova A, Hodam JR, Tolleson WH, et al. Retinoic acid suppresses human papillomavirus type 16 (HPV16)-mediated transformation of human keratinocytes and inhibits the expression of the HPV16 oncogenes. Diet and Cancer: Springer; 1994. p. 19-35. [view at publisher] [DOI] [PMID] [Google Scholar]
123. Shipley GD, Pittelkow MR, Wille JJ, Scott RE, Moses HL. Reversible inhibition of normal human prokeratinocyte proliferation by type β transforming growth factor-growth inhibitor in serum-free medium. Cancer research. 1986;46(4 Part 2):2068-71. [view at publisher] [Google Scholar]
124. Coffey RJ, Sipes NJ, Bascom CC, Graves-Deal R, Pennington CY, Weissman BE, et al. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer research. 1988;48(6):1596-602. [view at publisher] [Google Scholar]
125. Batova A, Danielpour D, Pirisi L, Creek K. Retinoic acid induces secretion of latent transforming growth factor beta 1 and beta 2 in normal and human papillomavirus type 16-immortalized human keratinocytes. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1992;3(11):763-72. [view at publisher] [Google Scholar]
126. Woodworth C, Notario V, DiPaolo J. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. Journal of virology. 1990;64(10):4767-75. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
127. Borger DR, Geslani G, Zyzak LL, Batova A, Engin TS, Pirisi L, et al. Retinoic acid resistance at late stages of human papillomavirus type 16-mediated transformation of human keratinocytes arises despite intact retinoid signaling and is due to a loss of sensitivity to transforming growth factor-β. Virology. 2000;270(2):397-407. [view at publisher] [DOI] [PMID] [Google Scholar]
128. Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL‐60 and U937 cell lines. Journal of leukocyte biology. 1985;37(4):407-22. [view at publisher] [DOI] [PMID] [Google Scholar]
129. Fujii N, Yokosawa N, Ishida S, Shirakawa S, Kubota T, Indoh T, et al. Investigation of IFN type-I receptor and IFN regulatory factor expression relating to induction of 2', 5'-oligoadenylate synthetase in cells persistently infected with the mumps virus. Microbiology and immunology. 1996;40(10):777-81. [view at publisher] [DOI] [PMID] [Google Scholar]
130. Indoh T, Shirakawa S, Kubota T, Yashiki T, Isogai E, Fujii N. Augmentation of interferon production after cell-differentiation of U937 cells by TPA. Microbiology and immunology. 1996;40(9):675-9. [view at publisher] [DOI] [PMID] [Google Scholar]
131. Hariya Y, Yokosawa N, Yonekura N, Kohama Gi, Fujii N. Mumps Virus Can Suppress the Effective Augmentation of HPC‐Induced Apoptosis by IFN‐γ through Disruption of IFN Signaling in U937 Cells. Microbiology and immunology. 2000;44(6):537-41. [view at publisher] [DOI] [PMID] [Google Scholar]
132. Soye KJ, Trottier C, Di Lenardo TZ, Restori KH, Reichman L, Miller WH, et al. In vitro inhibition of mumps virus by retinoids. Virology journal. 2013;10(1):337. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
133. Percario ZA, Giandomenico V, Fiorucci G, Chiantore MV, Vannucchi S, Hiscott J, et al. Retinoic acid is able to induce interferon regulatory factor 1 in squamous carcinoma cells via a STAT-1 independent signalling pathway. Cell Growth and Differentiation-Publication American Association for Cancer Research. 1999;10(4):263-70. [Google Scholar]
134. Arany I, Whitehead WE, Grattendick KJ, Ember IA, Tyring SK. Suppression of growth by all-trans retinoic acid requires prolonged induction of interferon regulatory factor 1 in cervical squamous carcinoma (SiHa) cells. Clin Diagn Lab Immunol. 2002;9(5):1102-6. [DOI] [PMID] [PMCID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






