Volume 12, Issue 3 (12-2024)
Jorjani Biomed J 2024, 12(3): 15-17 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khademi A, Mohammadi Z, Tohidi F. Investigating Crithidia spp. in ulcer smear of patients suspected of leishmaniasis in Aq-Qala, Golestan province, Northern Iran, 2019-2020. Jorjani Biomed J 2024; 12 (3) :15-17
URL: http://goums.ac.ir/jorjanijournal/article-1-1040-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1040-en.html
1- Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran
2- Department of Biology, Faculty of Sciences, Golestan University, Gorgan, Iran
3- Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,tohidi66@yahoo.ca
2- Department of Biology, Faculty of Sciences, Golestan University, Gorgan, Iran
3- Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,
Full-Text [PDF 406 kb]
(237 Downloads)
| Abstract (HTML) (1226 Views)
Discussion
Cutaneous leishmaniasis is one of the health and research priorities of the World Health Organization, especially in relation to developing countries. It has received special attention in Iran, with a focus on its various aspects. The disease is one of the most important infectious diseases in Golestan Province, northern Iran, and has caused significant health problems in society. In the present study, 117 of the smears (83.6%) were positive for Leishmania major parasite, three of the smears (2.1%) were positive for Leishmania tropica, and one of the smears (0.7%) was positive for both Crithidia and Leishmania major parasites. In other words, there is a significant relationship between Leishmania PCR and the type of ulcer, such as wet or dry (P-value < 0.05). Therefore, people with wet wounds are infected with Leishmania major, and people with dry wounds are infected with Leishmania tropica. In the study by Ghobakhloo et al., 92.8% of patients suspected of CL had only Leishmania major, 1.8% of patients had only Crithidia spp., and 5.4% of patients were infected with both Leishmania major and Crithidia spp. (15). Co-infection of Crithidia spp. in clinical cases of CL has been frequently reported in Iran. In Kalantari's study, Crithidia was detected in two CL patients who were chronically infected with L. major. In this study, there was one patient with concomitant Crithidia spp. and L. major infection. Another study by Ghobakhloo in 2019 showed that 50% of patients had Leishmania spp. and Crithidia spp. and were resistant to Glucantime (4). The increasing prevalence of Glucantime resistance and severity of cases, particularly in Iran, may be associated with the co-infection of Leishmania spp. and Crithidia spp. This concomitant infection could potentially explain the drug resistance observed in CL patients (17,18). In the study by Kalantari et al., 61.2% of Tatera indica were infected with Leishmania, and 2.4% were reported positive for Crithidia (16). In Doudi et al.'s study, 201 samples were isolated from suspected patients with CL who referred to the Leishmaniasis Research Center or other health centers in the vicinity of Isfahan. Of these, 33.3% of the samples were infected with Crithidia spp., and 58.3% were infected with Leishmania spp. (7). In the study by Brazesh et al., out of 66 patients with CL in Shiraz, 60 samples were infected with Leishmania major, and six samples were infected with Leishmania tropica. Furthermore, two samples were infected with both Leishmania and Crithidia spp. (19). In the study by Mirzapour et al., out of 70 patients with CL in Shiraz, 46 samples (65.7%), 17 samples (24.2%), and seven samples (10%) were infected with Leishmania major, Leishmania tropica, and Crithidia fasciculata, respectively (14). In some previous studies, Crithidia spp. have been frequently reported, likely because these studies were mostly conducted on rodents, where this parasite is commonly found in animals. In the present study, we found that 12.1% of the participants under 6 years old, 25.2% of the participants aged 7-19 years, 26.7% of the participants aged 20-40 years, and 35.6% of the participants over 40 years old had leishmaniasis. The Chi-square test showed that the frequency of L. major was the highest in all age groups; however, there was no statistically significant relationship between Leishmania and age groups (P-value > 0.05). In the study by Axmedovich et al., 20% of leishmaniasis patients were under 20 years old, 46.4% were 20-40 years old, and 32.8% were over 40 years old (1). In the present study, 68.5% of individuals were men, and 31.5% were women. Similarly, in the study by Axmedovich et al., 42.1% of leishmaniasis patients were women, and 57.9% were men (1). In the study by Khazaei et al., 55.1% of the patients were men, and 44.8% were women (2). In the study by Al-Khayat et al., 56.7% of Leishmania-positive samples were male, and 43.3% were female (3). In the same study by Al-Khayat et al., 57.6% of patients with dry wounds were men, and 48.6% were women. In addition, 51.4% of patients with wet wounds were men, and 42.4% were women (3). In this study, out of the 140 patient samples, 137 (97.8%) had wet ulcers, and three (2.2%) had dry ulcers. In addition, 67.9% of the patients with wet wounds were men, and 32.1% were women, while 100% of the patients with dry wounds were men. Al-Khayat, in 2019, reported that case severity features, based on characteristics such as the number, size, site, and type of lesions, were more pronounced in males compared to females (3). In this study, 85.7% of patients were positive for the Leishmania parasite, with 61.4% of the positive cases being males. In this respect, the results of our study conform to those of Al-Khayat. These differences may be attributed to immunological variations between genders, with males potentially experiencing increased parasitism. Females typically exhibit stronger immune responses, and the hormone testosterone has been found to have a disease-promoting effect (20,21). Another reason for the higher incidence in men compared to women could be the full coverage typically worn by women, especially in Iran. Furthermore, the existence of habits such as sleeping outdoors among men and their greater contact with environmental factors due to work reasons can be considered as factors contributing to the increase in their incidence (22). In the study by Khazaei et al., 62.7% of patients had upper limb ulcers, 24.8% had neck ulcers, 2.7% had trunk ulcers, and 22.7% had lower limb ulcers (2). In the present study, 63.6% of patients with facial wounds were men, and 36.4% were women. Among those with trunk wounds, 50% were men and 50% were women. In addition, 68.7% of patients with hand ulcers were male, while 31.3% were female. Similarly, 71.4% of patients with foot ulcers were male, and 28.6% were female. In Al-Khayat et al.'s study, 59.3% of patients with limb ulcers were male, and 40.7% were female. Among those with facial wounds, 48.5% were men, while 51.5% were women. In addition, 53.8% of those with trunk wounds were men, and 46.2% were women (3). The Chi-square test showed that PCR results for Leishmania major were the most frequent; however, no statistically significant correlation was found between PCR results and wound site (P-value > 0.05). Lesions of CL usually appear on uncovered and exposed parts of the body. Therefore, depending on the customs and clothing of the residents of different regions and the blood-feeding habits of sandflies, the organs that have the most wastes vary across different parts of the world and even within countries. In the present study, 60% of the patients were new cases, and 40% were other cases. In the study by Khazaei et al., 96.91% of cases were new cases (2). Moreover, in the present study, 27.8% of people with ulcers were urban residents, and 72.1% were rural residents. In the study by Khazaei et al., 62.6% of people with CL lived in cities, and 37.4% lived in villages (2). A limitation of this study is that, due to the low rate of Crithidia spp. positive samples, the potential interference of this parasite in response to patient treatment is unclear. This study was retrospective, and we had no discretion in selecting patients.
Conclusion
More studies should be conducted in the field of simultaneous infection of Leishmania and Crithidia in humans. In addition, research on the effect of leishmaniasis treatments on Crithidia spp. could be useful.
Acknowledgement
This research is extracted from Ali Khademi's medical thesis with project code: 112536. We are grateful to the Vice-Chancellor of Research and Technology of Golestan University of Medical Sciences for the financial support to conduct this research.
Funding sources
This research was funded by Golestan University of Medical Sciences, Iran.
Ethical statement
Approval was granted by the ethical committee of Golestan University of Medical Sciences, Gorgan, Iran, with ethics code IR.GOUMS.REC.1400.393.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
AKh collected the samples and conducted the experiments. ZM performed the molecular experiments. FT was responsible for conceptualization, methodology, analysis, writing the manuscript, and review and editing.
Full-Text: (155 Views)
Introduction
Leishmaniasis is a parasitic disease found in over 99 countries (1,2). Annually, 1.5 to 2 million individuals become infected with this disease (3). Leishmaniasis is usually observed in several forms, such as cutaneous, visceral, and mucocutaneous. Visceral leishmaniasis can even lead to death. Every year, about 350,000,000 people worldwide are exposed to this disease, and twelve million people are infected with it (4). For this reason, it is one of the six important diseases of tropical regions that the World Health Organization has recommended and supports for studying and conducting research on its various aspects (5). In Iran, cutaneous leishmaniasis (CL) and visceral leishmaniasis have been reported. CL is the predominant form (6). Recent studies have shown that Crithidia parasites have been found in the lesions of patients with CL (7,8). Many studies have shown that Crithidia can also infect the Leishmania culture medium (9). In addition, Crithidia has been identified in CL lesions in humans (10) and animals, such as rodents (11) and dogs (12). Interestingly, the gene sequence of ribosomal RNA (rRNA) in Crithidia species shows a high degree of similarity to Leishmania. Crithidia is a parasite transmitted by the bites of infected insects, such as sandflies or fleas (9,13). Golestan Province is located in northern Iran and is an endemic region for rural CL. One of the endemic areas of CL in this province is Aq-Qala City, which is located in the eastern part of Golestan Province. Recently, drug resistance has been observed in the treatment of patients with CL (14). The presence of co-infections of Crithidia and Leishmania can indeed lead to inadequate treatment and potentially result in severe complications for patients (15,16). This research aims to investigate the slides of patients with CL for the concurrent presence of Crithidia and Leishmania parasites.
Methods
In this retrospective descriptive study, 140 Giemsa-stained smears from patients suspected of CL, along with their demographic and epidemiological data, were collected from the archives of the laboratory at the Leishmaniasis Diagnostic Laboratory, Aq-Qala Health Center, Golestan Province, Iran, during 2019-2020. DNA was extracted from the Giemsa-stained smears, and PCR was used for the amplification of the ITS1 and GPDH genes in Leishmania and Crithidia spp., respectively.
DNA extraction and PCR
We extracted the DNA from the Giemsa-stained smears of patients suspected of CL using a DNA extraction kit (DENAzist, Mashhad, Iran) according to the manufacturer's protocol. CRF: 5´-TCCATGTGCGAGGACAACGTGCT-3´ and CRR: 3´-CGCGTCGTTGATGAAGTCGCT-5´ primers, based on the sequence of the GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) gene, were used to identify Crithidia spp. PCR was performed in a 25 µl volume, with 5 µl of DNA, 2 µl of 10 pmol primers, 12 µl of 2× master mix, and 6 µl of deionized water, following the PCR program: an initial step at 94°C for 5 min, 30 cycles at 94°C for 30 sec, annealing at 55°C for 1 min, extension at 72°C for 90 sec, and final extension at 72°C for 5 min to amplify an 800 bp fragment of the GPDH genes in Crithidia spp. For the detection of Leishmania and differentiation of L. major and L. tropica, primers LIN4R (F): 5´-GGGGTTGGTGTAAAATAGGG-3´ and LIN17(R): 5´-TTTGAACGGGATTTCTG-3´ were used to amplify the partial ITS1 gene. The PCR program was as follows: 95°C for 5 min, 35 cycles at 94°C for 30 sec, 52°C for 30 sec, 72°C for 45 sec, and 72°C for 8 min to amplify fragments of 650 bp for L. major and 760 bp for L. tropica. In these experiments, a mastermix solution, primers, and distilled water without DNA were used as negative controls, while Leishmania major and Leishmania tropica DNA were used as positive controls.
Statistical analysis
The results of the questionnaires were analyzed using SPSS version 25.0. Chi-square and Fisher's exact tests were used. P-values < 0.05 were considered significant.
Results
The result of the Leishmania
In our research, 140 patients suspected of CL were studied. PCR results showed that 117 patients (83.5%) were positive for L. major, three patients (2.1%) were positive for L. tropica, and 20 patients (14.2%) were negative for Leishmania parasite. Of the 140 patients, 44 (31.5%) were women, and 96 (68.5%) were men. The results showed that in the 20-40 age group, 33 patients (91.7%) were positive for L. major, while among those over 40 years old, 42 patients (87.5%) were positive for L. major. Three patients (One from each group) under six years old (5.9%), 7-19 years old (2.9%), and over 40 years old (2.1%) were infected with L. tropica. Our results showed that 84.6% of patients living in the city and 83.1% of those in the village were positive for L. major. Meanwhile, 5.1% of patients in the city and 0.9% of patients in the village were positive for L. tropica. Five patients (3.5%) had a history of treated leishmaniasis, and 51 patients (36.5%) had an unknown history of Leishmania infection. There was no significant relationship between Leishmania infection and the history of CL (P-value > 0.05). The appearance of wounds was wet (Ulcers with cerusite) in 137 people (97.8%) and dry (Ulcers without cerusite) in three people (2.2%). The results showed that 117 patients (85.4%) with wet wounds were infected with L. major. In addition, all three patients (100%) with dry wounds were infected with L. tropica. Eleven patients (7.8%) had cutaneous ulcers on the head and face, three patients (2.1%) had neck ulcers, 10 patients (7.1%) had trunk ulcers, 67 patients (47.8%) had upper limb ulcers, and 49 patients (35%) had lower limb ulcers. Chi-square test showed that PCR results of L. major are the most frequent. The result indicated that 74.7% of patients with one wound, 89.5% of patients with two wounds, 96.4% of patients with three wounds, 90.9% of patients with four wounds, and 100% of those with five or more wounds were positive for L. major (Figure 1).
The result of the Crithidia spp.
In evaluating the PCR results for Crithidia, among the 140 studied samples, only one female patient (0.7%) tested positive for Crithidia species. She was aged 7-19 (2.9%), had one wound (1.3%) on her foot (2%), and the wound was wet (0.7%), with no previous history of infection. This patient was a student and a city resident. The results showed that this patient was positive for Crithidia PCR and was also infected with L. major (Figure 2).
Leishmaniasis is a parasitic disease found in over 99 countries (1,2). Annually, 1.5 to 2 million individuals become infected with this disease (3). Leishmaniasis is usually observed in several forms, such as cutaneous, visceral, and mucocutaneous. Visceral leishmaniasis can even lead to death. Every year, about 350,000,000 people worldwide are exposed to this disease, and twelve million people are infected with it (4). For this reason, it is one of the six important diseases of tropical regions that the World Health Organization has recommended and supports for studying and conducting research on its various aspects (5). In Iran, cutaneous leishmaniasis (CL) and visceral leishmaniasis have been reported. CL is the predominant form (6). Recent studies have shown that Crithidia parasites have been found in the lesions of patients with CL (7,8). Many studies have shown that Crithidia can also infect the Leishmania culture medium (9). In addition, Crithidia has been identified in CL lesions in humans (10) and animals, such as rodents (11) and dogs (12). Interestingly, the gene sequence of ribosomal RNA (rRNA) in Crithidia species shows a high degree of similarity to Leishmania. Crithidia is a parasite transmitted by the bites of infected insects, such as sandflies or fleas (9,13). Golestan Province is located in northern Iran and is an endemic region for rural CL. One of the endemic areas of CL in this province is Aq-Qala City, which is located in the eastern part of Golestan Province. Recently, drug resistance has been observed in the treatment of patients with CL (14). The presence of co-infections of Crithidia and Leishmania can indeed lead to inadequate treatment and potentially result in severe complications for patients (15,16). This research aims to investigate the slides of patients with CL for the concurrent presence of Crithidia and Leishmania parasites.
Methods
In this retrospective descriptive study, 140 Giemsa-stained smears from patients suspected of CL, along with their demographic and epidemiological data, were collected from the archives of the laboratory at the Leishmaniasis Diagnostic Laboratory, Aq-Qala Health Center, Golestan Province, Iran, during 2019-2020. DNA was extracted from the Giemsa-stained smears, and PCR was used for the amplification of the ITS1 and GPDH genes in Leishmania and Crithidia spp., respectively.
DNA extraction and PCR
We extracted the DNA from the Giemsa-stained smears of patients suspected of CL using a DNA extraction kit (DENAzist, Mashhad, Iran) according to the manufacturer's protocol. CRF: 5´-TCCATGTGCGAGGACAACGTGCT-3´ and CRR: 3´-CGCGTCGTTGATGAAGTCGCT-5´ primers, based on the sequence of the GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) gene, were used to identify Crithidia spp. PCR was performed in a 25 µl volume, with 5 µl of DNA, 2 µl of 10 pmol primers, 12 µl of 2× master mix, and 6 µl of deionized water, following the PCR program: an initial step at 94°C for 5 min, 30 cycles at 94°C for 30 sec, annealing at 55°C for 1 min, extension at 72°C for 90 sec, and final extension at 72°C for 5 min to amplify an 800 bp fragment of the GPDH genes in Crithidia spp. For the detection of Leishmania and differentiation of L. major and L. tropica, primers LIN4R (F): 5´-GGGGTTGGTGTAAAATAGGG-3´ and LIN17(R): 5´-TTTGAACGGGATTTCTG-3´ were used to amplify the partial ITS1 gene. The PCR program was as follows: 95°C for 5 min, 35 cycles at 94°C for 30 sec, 52°C for 30 sec, 72°C for 45 sec, and 72°C for 8 min to amplify fragments of 650 bp for L. major and 760 bp for L. tropica. In these experiments, a mastermix solution, primers, and distilled water without DNA were used as negative controls, while Leishmania major and Leishmania tropica DNA were used as positive controls.
Statistical analysis
The results of the questionnaires were analyzed using SPSS version 25.0. Chi-square and Fisher's exact tests were used. P-values < 0.05 were considered significant.
Results
The result of the Leishmania
In our research, 140 patients suspected of CL were studied. PCR results showed that 117 patients (83.5%) were positive for L. major, three patients (2.1%) were positive for L. tropica, and 20 patients (14.2%) were negative for Leishmania parasite. Of the 140 patients, 44 (31.5%) were women, and 96 (68.5%) were men. The results showed that in the 20-40 age group, 33 patients (91.7%) were positive for L. major, while among those over 40 years old, 42 patients (87.5%) were positive for L. major. Three patients (One from each group) under six years old (5.9%), 7-19 years old (2.9%), and over 40 years old (2.1%) were infected with L. tropica. Our results showed that 84.6% of patients living in the city and 83.1% of those in the village were positive for L. major. Meanwhile, 5.1% of patients in the city and 0.9% of patients in the village were positive for L. tropica. Five patients (3.5%) had a history of treated leishmaniasis, and 51 patients (36.5%) had an unknown history of Leishmania infection. There was no significant relationship between Leishmania infection and the history of CL (P-value > 0.05). The appearance of wounds was wet (Ulcers with cerusite) in 137 people (97.8%) and dry (Ulcers without cerusite) in three people (2.2%). The results showed that 117 patients (85.4%) with wet wounds were infected with L. major. In addition, all three patients (100%) with dry wounds were infected with L. tropica. Eleven patients (7.8%) had cutaneous ulcers on the head and face, three patients (2.1%) had neck ulcers, 10 patients (7.1%) had trunk ulcers, 67 patients (47.8%) had upper limb ulcers, and 49 patients (35%) had lower limb ulcers. Chi-square test showed that PCR results of L. major are the most frequent. The result indicated that 74.7% of patients with one wound, 89.5% of patients with two wounds, 96.4% of patients with three wounds, 90.9% of patients with four wounds, and 100% of those with five or more wounds were positive for L. major (Figure 1).
The result of the Crithidia spp.
In evaluating the PCR results for Crithidia, among the 140 studied samples, only one female patient (0.7%) tested positive for Crithidia species. She was aged 7-19 (2.9%), had one wound (1.3%) on her foot (2%), and the wound was wet (0.7%), with no previous history of infection. This patient was a student and a city resident. The results showed that this patient was positive for Crithidia PCR and was also infected with L. major (Figure 2).
.PNG) Figure 1. PCR product electrophoresis from sample of patients Lane 1: DNA ladder marker 100bp Lane 2: Leishmania major positive control Lane 3, 6-11: Leishmania major positive samples Lane 4: Leishmania tropica positive control Lane 5: Negative control 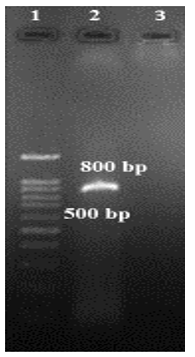 Figure 2. Gel electrophoresis of sample from the CL patient was Crithidia positive Lane 1: DNA ladder marker 100bp Lane 2: Positive sample (Crithidia spp.) 800bp Lane 3: Negative control |
Discussion
Cutaneous leishmaniasis is one of the health and research priorities of the World Health Organization, especially in relation to developing countries. It has received special attention in Iran, with a focus on its various aspects. The disease is one of the most important infectious diseases in Golestan Province, northern Iran, and has caused significant health problems in society. In the present study, 117 of the smears (83.6%) were positive for Leishmania major parasite, three of the smears (2.1%) were positive for Leishmania tropica, and one of the smears (0.7%) was positive for both Crithidia and Leishmania major parasites. In other words, there is a significant relationship between Leishmania PCR and the type of ulcer, such as wet or dry (P-value < 0.05). Therefore, people with wet wounds are infected with Leishmania major, and people with dry wounds are infected with Leishmania tropica. In the study by Ghobakhloo et al., 92.8% of patients suspected of CL had only Leishmania major, 1.8% of patients had only Crithidia spp., and 5.4% of patients were infected with both Leishmania major and Crithidia spp. (15). Co-infection of Crithidia spp. in clinical cases of CL has been frequently reported in Iran. In Kalantari's study, Crithidia was detected in two CL patients who were chronically infected with L. major. In this study, there was one patient with concomitant Crithidia spp. and L. major infection. Another study by Ghobakhloo in 2019 showed that 50% of patients had Leishmania spp. and Crithidia spp. and were resistant to Glucantime (4). The increasing prevalence of Glucantime resistance and severity of cases, particularly in Iran, may be associated with the co-infection of Leishmania spp. and Crithidia spp. This concomitant infection could potentially explain the drug resistance observed in CL patients (17,18). In the study by Kalantari et al., 61.2% of Tatera indica were infected with Leishmania, and 2.4% were reported positive for Crithidia (16). In Doudi et al.'s study, 201 samples were isolated from suspected patients with CL who referred to the Leishmaniasis Research Center or other health centers in the vicinity of Isfahan. Of these, 33.3% of the samples were infected with Crithidia spp., and 58.3% were infected with Leishmania spp. (7). In the study by Brazesh et al., out of 66 patients with CL in Shiraz, 60 samples were infected with Leishmania major, and six samples were infected with Leishmania tropica. Furthermore, two samples were infected with both Leishmania and Crithidia spp. (19). In the study by Mirzapour et al., out of 70 patients with CL in Shiraz, 46 samples (65.7%), 17 samples (24.2%), and seven samples (10%) were infected with Leishmania major, Leishmania tropica, and Crithidia fasciculata, respectively (14). In some previous studies, Crithidia spp. have been frequently reported, likely because these studies were mostly conducted on rodents, where this parasite is commonly found in animals. In the present study, we found that 12.1% of the participants under 6 years old, 25.2% of the participants aged 7-19 years, 26.7% of the participants aged 20-40 years, and 35.6% of the participants over 40 years old had leishmaniasis. The Chi-square test showed that the frequency of L. major was the highest in all age groups; however, there was no statistically significant relationship between Leishmania and age groups (P-value > 0.05). In the study by Axmedovich et al., 20% of leishmaniasis patients were under 20 years old, 46.4% were 20-40 years old, and 32.8% were over 40 years old (1). In the present study, 68.5% of individuals were men, and 31.5% were women. Similarly, in the study by Axmedovich et al., 42.1% of leishmaniasis patients were women, and 57.9% were men (1). In the study by Khazaei et al., 55.1% of the patients were men, and 44.8% were women (2). In the study by Al-Khayat et al., 56.7% of Leishmania-positive samples were male, and 43.3% were female (3). In the same study by Al-Khayat et al., 57.6% of patients with dry wounds were men, and 48.6% were women. In addition, 51.4% of patients with wet wounds were men, and 42.4% were women (3). In this study, out of the 140 patient samples, 137 (97.8%) had wet ulcers, and three (2.2%) had dry ulcers. In addition, 67.9% of the patients with wet wounds were men, and 32.1% were women, while 100% of the patients with dry wounds were men. Al-Khayat, in 2019, reported that case severity features, based on characteristics such as the number, size, site, and type of lesions, were more pronounced in males compared to females (3). In this study, 85.7% of patients were positive for the Leishmania parasite, with 61.4% of the positive cases being males. In this respect, the results of our study conform to those of Al-Khayat. These differences may be attributed to immunological variations between genders, with males potentially experiencing increased parasitism. Females typically exhibit stronger immune responses, and the hormone testosterone has been found to have a disease-promoting effect (20,21). Another reason for the higher incidence in men compared to women could be the full coverage typically worn by women, especially in Iran. Furthermore, the existence of habits such as sleeping outdoors among men and their greater contact with environmental factors due to work reasons can be considered as factors contributing to the increase in their incidence (22). In the study by Khazaei et al., 62.7% of patients had upper limb ulcers, 24.8% had neck ulcers, 2.7% had trunk ulcers, and 22.7% had lower limb ulcers (2). In the present study, 63.6% of patients with facial wounds were men, and 36.4% were women. Among those with trunk wounds, 50% were men and 50% were women. In addition, 68.7% of patients with hand ulcers were male, while 31.3% were female. Similarly, 71.4% of patients with foot ulcers were male, and 28.6% were female. In Al-Khayat et al.'s study, 59.3% of patients with limb ulcers were male, and 40.7% were female. Among those with facial wounds, 48.5% were men, while 51.5% were women. In addition, 53.8% of those with trunk wounds were men, and 46.2% were women (3). The Chi-square test showed that PCR results for Leishmania major were the most frequent; however, no statistically significant correlation was found between PCR results and wound site (P-value > 0.05). Lesions of CL usually appear on uncovered and exposed parts of the body. Therefore, depending on the customs and clothing of the residents of different regions and the blood-feeding habits of sandflies, the organs that have the most wastes vary across different parts of the world and even within countries. In the present study, 60% of the patients were new cases, and 40% were other cases. In the study by Khazaei et al., 96.91% of cases were new cases (2). Moreover, in the present study, 27.8% of people with ulcers were urban residents, and 72.1% were rural residents. In the study by Khazaei et al., 62.6% of people with CL lived in cities, and 37.4% lived in villages (2). A limitation of this study is that, due to the low rate of Crithidia spp. positive samples, the potential interference of this parasite in response to patient treatment is unclear. This study was retrospective, and we had no discretion in selecting patients.
Conclusion
More studies should be conducted in the field of simultaneous infection of Leishmania and Crithidia in humans. In addition, research on the effect of leishmaniasis treatments on Crithidia spp. could be useful.
Acknowledgement
This research is extracted from Ali Khademi's medical thesis with project code: 112536. We are grateful to the Vice-Chancellor of Research and Technology of Golestan University of Medical Sciences for the financial support to conduct this research.
Funding sources
This research was funded by Golestan University of Medical Sciences, Iran.
Ethical statement
Approval was granted by the ethical committee of Golestan University of Medical Sciences, Gorgan, Iran, with ethics code IR.GOUMS.REC.1400.393.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
AKh collected the samples and conducted the experiments. ZM performed the molecular experiments. FT was responsible for conceptualization, methodology, analysis, writing the manuscript, and review and editing.
Type of Article: Original article |
Subject:
Epidemiology
Received: 2024/07/2 | Accepted: 2024/09/25 | Published: 2025/05/21
Received: 2024/07/2 | Accepted: 2024/09/25 | Published: 2025/05/21
References
1. Axmedovich MF, Ikromovich LI, Hamza o'g'li OJ. Statistics of the incidence of cutaneous leishmaniasis in the Bukhara region, depending on age, gender and region. Middle European Sci Bull. 2021;17:373-7. [View at Publisher] [DOI] [Google Scholar]
2. Khazaei S, Hafshejani AM, Saatchi M, Salehiniya H, Nematollahi S. Epidemiological aspects of cutaneous leishmaniasis in Iran. Arch. Clin Infect Dis. 2015;10(3):e28511. [View at Publisher] [DOI] [Google Scholar]
3. Al-Khayat Z, Agha N, Alharmni K. Gender differences in the severity and features of lesions among cutaneous Leishmaniasis patients. J Contemp Med Sci. 2019;5(6):336-42. [View at Publisher] [DOI] [Google Scholar]
4. WHO. Global leishmaniasis update, 2006-2015: A turning point in leishmaniasis surveillance. Wkly Epidemiol Rec. 2017;92(38):557-65. [View at Publisher] [Google Scholar]
5. Norouzinezhad F, Ghaffari F, Norouzinejad A, Kaveh F, Gouya MM. Cutaneous leishmaniasis in Iran: Results from an epidemiological study in urban and rural province. Asian Pac J Trop Biomed. 2016;6(7):614-9. [View at Publisher] [DOI] [Google Scholar]
6. Kalantari M, Motazedian MH, Asgari Q, Soltani A, Mohammadpour I, Azizi K. DNA-based detection of Leishmania and Crithidia species isolated from humans in cutaneous and post-kala-azar dermal leishmaniasis from Shiraz and Kharameh, southern Iran. J Vector Borne Dis. 2020;57(1):52-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Doudi M, Karami M, Eslami G, Setorki M. A study of genetic polymorphism of Crithidia in Isfahan, Iran. Zahedan J Res Med Sci (ZJRMS). 2015;17(5): 971 [View at Publisher] [DOI] [Google Scholar]
8. Spotin A, Rouhani S, Parvizi P. The associations of Leishmania major and Leishmania tropica aspects by focusing their morphological and molecular features on clinical appearances in Khuzestan province, Iran. Biomed Res Int. 2014:2014:913510. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Mundim MH, Roitman I, HermansMA, Kitajima EW. Simple nutrition of Crithidia deanei, a reduviid trypanosomatid with an endosymbiont. J Protozool. 1974;21(4):518-21. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Bordbar A, Parvizi P. High infection frequency, low diversity of Leishmania major and first detection of Leishmania turanica in human in northern Iran. Acta Trop. 2014:133:69-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Moghaddam Y, Ziaei Hezarjaribi H, Fakhar M , Pagheh AS , Saberi R , Sharbatkhori M, et al. Al. Molecular detection of Critidia fasciculata and other blood parasites in Rhombomis opimus from northern Iran as endemic area. Internation J of Molecul and Clinic Microbio. 2021;11(2):1548-55. [View at Publisher] [Google Scholar]
12. Fakhar M, Derakhshani-nia M, Gohardehi Sh, Karamian M, Ziaei Hezarjaribi H, Mohebali M, et al. Domestic dogs carriers of Leishmania infantum, Leishmania tropica and Crithidia fasciculata as potential reservoirs for human visceral leishmaniasis in northeastern Iran. Vet Med Sci. 2022;8(6):2329-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Marmur J, Cahoon M, Shimura Y, Vogel HJ. Deoxyribonucleic acid type attributable to a bacterial endosymbiote in the protozoon Crithidia (Strigomonas) oncopelti. Nature. 1963;197(4873):1228-9. [View at Publisher] [DOI] [Google Scholar]
14. Mirzapour A, Badirzadeh A, Ashrafmansouri M, Behniafar H, Norouzi M, Azizi H, et al. Super infection of cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica to Crithidia fasciculata in Shiraz, Iran. Iran J Public Health. 2019;48(12): 2285-92. [View at Publisher] [DOI] [Google Scholar]
15. Ghobakhloo N, Motazedian MH, Naderi S, Ebrahimi S. Isolation of Crithidia spp. from lesions of immunocompetent patients with suspected cutaneous leishmaniasis in Iran. Trop Med Int Health. 2019;24(1):116-26. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Kalantari M, Motazedian MH, Asgari Q, Mohammadpour I, Soltani A, Azizi K. Co-detection and isolation of Leishmania and Crithidia among naturally infected Tatera indica (Rodentia: Muridae) in Fars province, southern Iran. Asian Pac J Trop Biomed. 2018;8(5):279-84. [View at Publisher] [DOI] [Google Scholar]
17. Alotaibi A, Ebiloma GU, Williams R, Alenezi S, Donachie AM, Guillaume S and Et al. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci Rep. 2019;9(1):11364. [view at publisher] [DOI] [PMID] [Google Scholar]
18. Moghaddam Y, Ziaei Hezarjaribi H, Fakhar M, Pagheh AS, Saberi R, Nazar E, et al. New Epidemiological Feature of Cutaneous Leishmaniasis in an Endemic Focus in Golestan Province. Sci J Kurdistan Univ Med Sci. 2022;27(3):77-88. [View at Publisher] [DOI] [Google Scholar]
19. Barazesh A, Motazedian MH, Fouladvand M, Hatam G, Tajbakhsh S, Ebrahimi S, et al. Molecular identification of species caused cutaneous leishmaniasis in southern zone of Iran. J Arthropod Borne Dis. 2019;13(2):198-205. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Lima ÁL, de Lima ID, Coutinho JF, de Sousa ÚP, Rodrigues MA, Wilson ME, et al. Changing epidemiology of visceral leishmaniasis in northeastern Brazil: a 25-year follow-up of an urban outbreak. Trans R Soc Trop Med Hyg. 2017;111(10):440-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Alotaibi A, Ebiloma GU, Williams R, Alenezi S, Donachie AM, Guillaume S, et al. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci Rep. 2019;9(1):11364. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Tohidi F, Borghae A. Cutaneous leishmaniasis Parasite Identification via PCR in the Infected Areas in Golestan Province. Knowledge & Health. 2011;6(2):26-31. [View at Publisher] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






