Volume 12, Issue 1 (10-2024)
Jorjani Biomed J 2024, 12(1): 10-13 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi A, Taheri Kalani A, Omidi M. Impact of high-fat diet and resistance training on oxidative stress and cardiac health in rats. Jorjani Biomed J 2024; 12 (1) :10-13
URL: http://goums.ac.ir/jorjanijournal/article-1-1028-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1028-en.html
1- Department of Exercise Physiology, Ilam Branch, Islamic Azad University, Ilam, Iran
2- Department of Exercise Physiology, Ilam Branch, Islamic Azad University, Ilam, Iran ,htaheriedu@gmail.com
2- Department of Exercise Physiology, Ilam Branch, Islamic Azad University, Ilam, Iran ,
Keywords: High-fat diet, Resistance Training, Oxidative Stress, Antioxidants, Cardiac Tissue, Animal Models
Full-Text [PDF 441 kb]
(3233 Downloads)
| Abstract (HTML) (10270 Views)
Full-Text: (1866 Views)
Introduction
Changing the lifestyle towards low daily physical activities and sedentary life, along with the consumption of high-fat diets (HFD), can threaten the health of individuals and society (1). During mitochondrial β-oxidation of fatty acids, oxidized cofactors (FAD and NAD+) first convert into reduced cofactors (FADH2 and NADH) and re-oxidize by the mitochondrial respiratory chain. After NADH and FADH2 reoxidation, electrons transfer to the first complexes of the respiratory chain and combine with oxygen and protons to form water. Some of these intermediates are converted into reactive oxygen species (ROS) and superoxide anion radicals by reacting with oxygen (2). Therefore, HFD feeding promotes the beta-oxidation of fatty acids in the mitochondria and accumulates ROS by increasing the flow of excess electrons using cytochrome c oxidase (3). When there is an imbalance between the production of ROS and endogenous antioxidant defense mechanisms, oxidative stress occurs (4). Cardiac cells are vulnerable to oxidative stress because they have fewer antioxidant enzymes to remove ROS (5). Increased oxidative stress may be effective in the pathogenesis of cardiovascular diseases (6). Clinical and experimental studies have demonstrated that these diseases cause oxidative damage in cardiac and aorta cells by increasing the formation of free radicals or reducing antioxidant defense (7). Also, it has been reported that feeding with HFD in the long term through the reduction of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) and the increase of malondialdehyde (MDA) in humans and animals, can lead to oxidative stress and cell damage (8-10).
Regular exercise has many health benefits and is associated with a decrease in all-cause mortality in humans (11). At the same time, it has been found that generating free radicals during many types of exercise (Prolonged endurance exercise, resistance exercise, high-intensity anaerobic exercise, and eccentric exercise) causes oxidative damage to cell structures (12). Recent studies have changed attitudes about exercise-induced oxidative stress. Although acute exercise can trigger damage to cellular structures by promoting the formation of ROS, chronic exercise, by up-regulating cellular antioxidant mechanisms and modulating the production of oxidants, plays a useful role in controlling gene expression, regulating cell signaling pathways, and muscle adaptations (13,14). It seems that investigating the effects of various pharmaceutical and non-pharmacological approaches to suppress or attenuate the parameters related to oxidative stress is highly important. However, there are few controlled studies in this area.
Although resistance training (RT) is part of the exercise recommended by the American Heart and Diabetes Association (15), there is limited information about the effect of this type of training on oxidative stress markers and the mechanisms involved. It has been shown that RT results in a redox balance in cardiac tissue by an increase in antioxidant enzyme levels (8,16). However, changes in oxidative stress biomarkers and mechanisms involved following HFD and exercise training are mainly unknown. It has been shown that the ability to neutralize oxidant species differs in various models of HFD and training (17).
In recent decades, in line with the rapid prevalence of non-communicable and chronic diseases, researchers have considered oxidative stress as a common mechanism in these diseases (18). Therefore, investigating the effects of various pharmacological and non-pharmacological approaches in controlling or ameliorating the parameters related to oxidative stress is highly important. Based on the existing knowledge, there is no clear information about the effects of exercise and nutritional interventions on the balance of oxidative-antioxidant stress of the heart. Therefore, this study aimed to evaluate the impact of HFD and RT on oxidative stress biomarkers and cardiac health in rats.
Methods
Animals
Twenty-one male Wistar rats aged 12- 14 weeks (200- 300 g) were obtained from the Pasteur Institute and transferred to an animal laboratory. After one week of familiarization with the laboratory environment, animals were randomly and equally assigned into the following groups (n=7): control (CTRL), which were fed for 23 weeks by the standard food and did not have any exercise, HFD, which were fed for 23 weeks by a high-fat diet and did not have any exercise, and HFD+RT, which consumed a high-fat diet during 23 weeks and also performed a resistance training protocol in the last eight weeks. Rats were housed in a 22±2°C and humidity of 45% room with a 12:12 hours light/dark photoperiod. Animals' water and food were checked daily, and standard water and food for each group were provided.
Diets
The standard diet for the CTRL group contained 4.30 kcal/g with 3.87% fat (Soy oil), 17.46% casein protein, 68.7% carbohydrates, 8.97% minerals, and 1% vitamins. The animals in the HFD (Total calories composed of 43% carbohydrate, 40% fat, and 17% protein) groups first received 20% fructose by gavage for five weeks, then 10% fructose was added every two weeks until the 9th week. From the beginning of the 10th week to the end of the 15th week, they received 50% fructose. Next, for four weeks (from the beginning of the 16th to the end of the 19th week), carbon tetrachloride (CCl4) 0.1 ml/kg/day dissolved in olive oil at a ratio of 1 to 6, and in the last four weeks, the intraperitoneal injection of CCl4 was stopped. Only olive oil was given to them as gavage (19). Previous studies have demonstrated that CCl4 combined with an HFD and fructose can induce oxidative stress, inflammation, and apoptosis (20).
RT protocol
The resistance training protocol included eight weeks and three sessions per week of climbing a 1-m-high homemade ladder with 26 steps at a distance of 4 cm, inclined at 80°. During the adaptation period, the rats spent seven days climbing the ladder without any load to become familiar with it. The initial load attached to each animal's tail was 30% of its body mass and increased progressively to 100% after 8 weeks (Table 1) (21). To determine the accurate load, the body mass of the animals was measured once every four days. Only touching and rubbing the animals' tails stimulated them to perform the exercises.
Sampling method from the cardiac tissue and measurement of the variables sampling was done at least 48 hours after the last training session to eliminate the acute effects of exercise. First, the animals were anesthetized by intraperitoneal injection of a combination of ketamine (70 mg/kg) and xylazine (3-5 mg/kg). The cardiac tissue of the rats was rapidly separated, weighed, and washed with cold saline, frozen in liquid nitrogen, and stored at -80 °C for further analysis. Next, the extracted tissue was homogenized with 17 mM phosphate buffer at a speed of 8000 rpm. Cardiac levels of SOD and GPX enzymes, total antioxidant capacity (TAC), and MDA were measured using a Randox kit (Mindray vs480, UK) by colorimetric method.
Statistical analysis
First, data normality was evaluated by the Shapiro-Wilk test. Then, one-way analysis of variance (ANOVA) was used to compare the mean values of variables between groups. When the ANOVA detected significant differences among groups, the Tukey post hoc test was used to identify where those differences occurred. The level of significance was set at P<0.05.
Results
Table 2 shows the body and cardiac mass of the animals after treatments. The one-way ANOVA results showed a significant difference in body mass between the groups (P<0.05). Tukey's post hoc test showed that the body mass of animals in the HFD group was significantly higher than the CTRL and HFD+RT groups (Both P=0.0001), but there was no significant difference between HFD+RT (P=0.613) and CTRL. Also, there was a significant difference in cardiac mass between the three groups (P=0.001). Tukey's post hoc test showed a significant increase in cardiac mass in the HFD+RT compared to the HFD group (P=0.001), but there was no significant difference between HFD+RT (P=0.283) and HFD (P=0.196) when compared with the CTRL group.
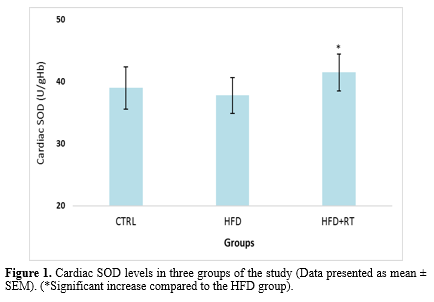
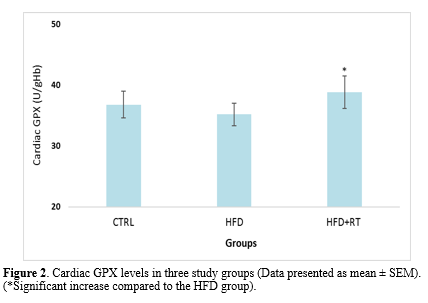
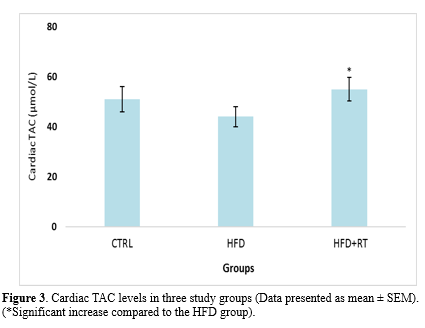
Data analysis showed that there was a significant difference in the cardiac SOD (F=5.072, P=0.019), GPX (F=4.504, P=0.026), and TAC (F=3.914, P=0.038) levels between the three groups. Results in Figures 1, 2, and 3 demonstrate a significant increase in cardiac SOD (P=0.021), GPX (P=0.024), and TAC (P=0.041) levels in HFD+RT compared to the HFD group. However, there was no significant difference between cardiac SOD, GPX, and TAC levels in the CTRL with the HFD (P=0.484, P=0.707, and P=0.653, respectively) and HFD+RT (P=0.136, P=0.209 and P=0.194; respectively) groups.
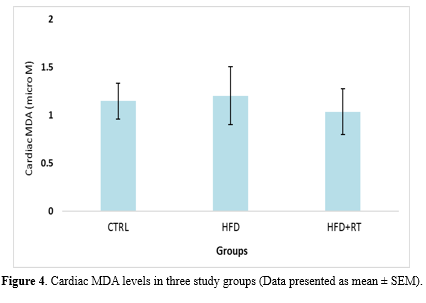
Cardiac MDA levels were not significantly altered in three of the groups used in the present study (F=0.864, P=0.438) (Figure 4).
Discussion
In this study, we demonstrated that HFD had no significant effect on the level of oxidative stress biomarkers (SOD, GPX, TAC, and MDA levels) in the cardiac tissue of rats. Although eight weeks of RT increased the levels of SOD, GPX enzymes, and TAC, it had no effect on MDA levels of rats fed HFD. Moreover, the decrease in body mass and increase in cardiac mass in the RT+HFD group compared to HFD demonstrated that exercise and diet interventions had exerted the expected effects on the animals.
In previous studies, consumption of an HFD in animal models was associated with increased oxidative stress biomarkers (Decrease in antioxidant enzymes and increase in lipid peroxidation) (8-10,22), which are different from our results. Skrzep et al. (2020) demonstrated that two months of HFD in rats caused a decrease in GPX and increased MDA in heart tissue (22). Also, following eight weeks of HFD in Wistar rats, a reduction in SOD, CAT, and GSH and an increase in MDA levels were reported (10). Another study reported that cardiac cells are susceptible to weight gain through elevating oxidative stress (8). The discrepancy between the findings in this study and other studies can be due to differences in the assessment method (Colorimetric vs. ELISA or spectrophotometric) and diet type (Fat content in diet). It seems that oxidative stress is caused by diets of more than eight weeks that provide at least 50% of calories from fat (9,10,22). The HFD applied in this study provided only 40% of calories from fat.
According to the results of the current study, the cardiac SOD, GPX, and TAC levels increased significantly after eight weeks of RT. The effects of resistance training on cardiac oxidative stress biomarkers have received less attention than aerobic training. Consistent with the findings of the current study, Effting et al. (2019) reported that eight weeks of RT improved antioxidant systems in the cardiac tissue of rats fed with an HFD (8). Similarly, after eight weeks of RT in rats with high blood pressure, an increase in the antioxidant enzymes such as GPX and SOD was reported (16). Moreover, it has been reported that RT increased antioxidant capacity in the skeletal muscle of infarcted rats (23,24). The similarity of the results is likely due to using the same training protocols.
Regular exercise training increases the presence of mitochondrial uncoupling protein 2 (UCP2) in the mitochondrial membrane of the heart. This reduces electron leakage and decreases the formation of free radicals (25). Also, exercise increases the gene expression of antioxidant enzymes, which increases the antioxidant capacity and reduces oxidative stress due to the neutralization of free radicals (26). SOD is the first antioxidant enzyme in the body against free radicals and eliminates superoxide anions generated by NADPH oxidase to form oxygen and hydrogen peroxide. Therefore, GPX not only removes hydrogen peroxide but also prevents the production of other harmful free radicals such as hydroxyl radicals (27). Indeed, the GPX enzyme has more protective effects against oxidative damage than SOD, because the dismutation of superoxide anion by the SOD enzyme may increase hydrogen peroxide (12). Therefore, GPX is more effective in protecting cells, tissues, and organs against oxidative damage compared to SOD. In addition, GPX is present in cardiac tissue, especially cytosolic and mitochondrial parts (28). These pieces of evidence indicate that the GPX enzyme is of special importance as a defense mechanism in heart tissue. Based on the results obtained in the present study, RT protects against oxidative damage of cardiac tissue against free radicals by increasing SOD, GPX and TAC levels. RT seems to reduce ROS production in the mitochondrial respiratory chain by increasing muscle mass and changing energy substrates. As a result, the need to use antioxidant systems to suppress ROS is reduced, leading to an increase in antioxidant enzymes.
With the main objective of investigating the effects of RT on oxidative damage in the cardiac tissue induced by the HFD, we evaluated the levels of MDA, a byproduct of lipoperoxidation. We did not observe differences in this marker between groups. Gomes et al. (2020) also confirmed that 12 weeks of RT had no effect on the lipid hydroperoxide concentration (An oxidative stress biomarker) in the gastrocnemius muscle of rats with infarction (23). Moreover, 10 weeks of RT did not change the activity of antioxidant enzymes and lipid peroxidation markers of the liver tissue of ovariectomized rats (29). However, another study has reported a decreased MDA level in rat cardiac tissues following feeding HFD for 26 weeks (8). Moreover, after eight weeks of RT in hypertensive rats, a decrease in lipid peroxidation (Chemiluminescence) and protein oxidation of cardiac tissue was reported (16). It can be concluded that the stressor factors in this study were not enough to damage the cell membrane and increase MDA levels. Nevertheless, due to the difference in the methodology of the conducted research, other controlled studies with a similar design should be performed.
Conclusion
In the present study, cardiac SOD, GPX, and TAC levels were improved, thereby improving antioxidant defense, which may prevent lipid peroxidation and MDA accumulation in rats fed an HFD. RT used in this study modulated the oxidative stress in the cardiac tissue of rats fed with an HFD, thereby reducing obesity-related health complications. Therefore, performing RT is an effective non-pharmacological approach to reducing oxidative stress in the cardiac tissue. However, for more accurate conclusions, it is suggested that more studies be conducted in this field.
Acknowledgement
No acknowledgment.
Funding sources
This research received no external funding.
Ethical statement
All animal studies were performed following the standard ethical instructions for working with laboratory animals approved by the research ethics committee of the Sport Sciences Research Institute (Ethical code: IR.SSRI.REC.1401.1909).
Conflicts of interest
There is no conflict of interest.
Author contributions
All authors contributed to developing the protocol, data abstraction, and manuscript preparation.
Changing the lifestyle towards low daily physical activities and sedentary life, along with the consumption of high-fat diets (HFD), can threaten the health of individuals and society (1). During mitochondrial β-oxidation of fatty acids, oxidized cofactors (FAD and NAD+) first convert into reduced cofactors (FADH2 and NADH) and re-oxidize by the mitochondrial respiratory chain. After NADH and FADH2 reoxidation, electrons transfer to the first complexes of the respiratory chain and combine with oxygen and protons to form water. Some of these intermediates are converted into reactive oxygen species (ROS) and superoxide anion radicals by reacting with oxygen (2). Therefore, HFD feeding promotes the beta-oxidation of fatty acids in the mitochondria and accumulates ROS by increasing the flow of excess electrons using cytochrome c oxidase (3). When there is an imbalance between the production of ROS and endogenous antioxidant defense mechanisms, oxidative stress occurs (4). Cardiac cells are vulnerable to oxidative stress because they have fewer antioxidant enzymes to remove ROS (5). Increased oxidative stress may be effective in the pathogenesis of cardiovascular diseases (6). Clinical and experimental studies have demonstrated that these diseases cause oxidative damage in cardiac and aorta cells by increasing the formation of free radicals or reducing antioxidant defense (7). Also, it has been reported that feeding with HFD in the long term through the reduction of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) and the increase of malondialdehyde (MDA) in humans and animals, can lead to oxidative stress and cell damage (8-10).
Regular exercise has many health benefits and is associated with a decrease in all-cause mortality in humans (11). At the same time, it has been found that generating free radicals during many types of exercise (Prolonged endurance exercise, resistance exercise, high-intensity anaerobic exercise, and eccentric exercise) causes oxidative damage to cell structures (12). Recent studies have changed attitudes about exercise-induced oxidative stress. Although acute exercise can trigger damage to cellular structures by promoting the formation of ROS, chronic exercise, by up-regulating cellular antioxidant mechanisms and modulating the production of oxidants, plays a useful role in controlling gene expression, regulating cell signaling pathways, and muscle adaptations (13,14). It seems that investigating the effects of various pharmaceutical and non-pharmacological approaches to suppress or attenuate the parameters related to oxidative stress is highly important. However, there are few controlled studies in this area.
Although resistance training (RT) is part of the exercise recommended by the American Heart and Diabetes Association (15), there is limited information about the effect of this type of training on oxidative stress markers and the mechanisms involved. It has been shown that RT results in a redox balance in cardiac tissue by an increase in antioxidant enzyme levels (8,16). However, changes in oxidative stress biomarkers and mechanisms involved following HFD and exercise training are mainly unknown. It has been shown that the ability to neutralize oxidant species differs in various models of HFD and training (17).
In recent decades, in line with the rapid prevalence of non-communicable and chronic diseases, researchers have considered oxidative stress as a common mechanism in these diseases (18). Therefore, investigating the effects of various pharmacological and non-pharmacological approaches in controlling or ameliorating the parameters related to oxidative stress is highly important. Based on the existing knowledge, there is no clear information about the effects of exercise and nutritional interventions on the balance of oxidative-antioxidant stress of the heart. Therefore, this study aimed to evaluate the impact of HFD and RT on oxidative stress biomarkers and cardiac health in rats.
Methods
Animals
Twenty-one male Wistar rats aged 12- 14 weeks (200- 300 g) were obtained from the Pasteur Institute and transferred to an animal laboratory. After one week of familiarization with the laboratory environment, animals were randomly and equally assigned into the following groups (n=7): control (CTRL), which were fed for 23 weeks by the standard food and did not have any exercise, HFD, which were fed for 23 weeks by a high-fat diet and did not have any exercise, and HFD+RT, which consumed a high-fat diet during 23 weeks and also performed a resistance training protocol in the last eight weeks. Rats were housed in a 22±2°C and humidity of 45% room with a 12:12 hours light/dark photoperiod. Animals' water and food were checked daily, and standard water and food for each group were provided.
Diets
The standard diet for the CTRL group contained 4.30 kcal/g with 3.87% fat (Soy oil), 17.46% casein protein, 68.7% carbohydrates, 8.97% minerals, and 1% vitamins. The animals in the HFD (Total calories composed of 43% carbohydrate, 40% fat, and 17% protein) groups first received 20% fructose by gavage for five weeks, then 10% fructose was added every two weeks until the 9th week. From the beginning of the 10th week to the end of the 15th week, they received 50% fructose. Next, for four weeks (from the beginning of the 16th to the end of the 19th week), carbon tetrachloride (CCl4) 0.1 ml/kg/day dissolved in olive oil at a ratio of 1 to 6, and in the last four weeks, the intraperitoneal injection of CCl4 was stopped. Only olive oil was given to them as gavage (19). Previous studies have demonstrated that CCl4 combined with an HFD and fructose can induce oxidative stress, inflammation, and apoptosis (20).
RT protocol
The resistance training protocol included eight weeks and three sessions per week of climbing a 1-m-high homemade ladder with 26 steps at a distance of 4 cm, inclined at 80°. During the adaptation period, the rats spent seven days climbing the ladder without any load to become familiar with it. The initial load attached to each animal's tail was 30% of its body mass and increased progressively to 100% after 8 weeks (Table 1) (21). To determine the accurate load, the body mass of the animals was measured once every four days. Only touching and rubbing the animals' tails stimulated them to perform the exercises.
Table 1. RT protocol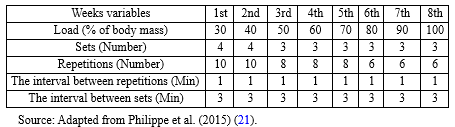 |
Statistical analysis
First, data normality was evaluated by the Shapiro-Wilk test. Then, one-way analysis of variance (ANOVA) was used to compare the mean values of variables between groups. When the ANOVA detected significant differences among groups, the Tukey post hoc test was used to identify where those differences occurred. The level of significance was set at P<0.05.
Results
Table 2 shows the body and cardiac mass of the animals after treatments. The one-way ANOVA results showed a significant difference in body mass between the groups (P<0.05). Tukey's post hoc test showed that the body mass of animals in the HFD group was significantly higher than the CTRL and HFD+RT groups (Both P=0.0001), but there was no significant difference between HFD+RT (P=0.613) and CTRL. Also, there was a significant difference in cardiac mass between the three groups (P=0.001). Tukey's post hoc test showed a significant increase in cardiac mass in the HFD+RT compared to the HFD group (P=0.001), but there was no significant difference between HFD+RT (P=0.283) and HFD (P=0.196) when compared with the CTRL group.
Table 2. Body and cardiac mass (g) of the groups after treatments |
Cardiac MDA levels were not significantly altered in three of the groups used in the present study (F=0.864, P=0.438) (Figure 4).




Discussion
In this study, we demonstrated that HFD had no significant effect on the level of oxidative stress biomarkers (SOD, GPX, TAC, and MDA levels) in the cardiac tissue of rats. Although eight weeks of RT increased the levels of SOD, GPX enzymes, and TAC, it had no effect on MDA levels of rats fed HFD. Moreover, the decrease in body mass and increase in cardiac mass in the RT+HFD group compared to HFD demonstrated that exercise and diet interventions had exerted the expected effects on the animals.
In previous studies, consumption of an HFD in animal models was associated with increased oxidative stress biomarkers (Decrease in antioxidant enzymes and increase in lipid peroxidation) (8-10,22), which are different from our results. Skrzep et al. (2020) demonstrated that two months of HFD in rats caused a decrease in GPX and increased MDA in heart tissue (22). Also, following eight weeks of HFD in Wistar rats, a reduction in SOD, CAT, and GSH and an increase in MDA levels were reported (10). Another study reported that cardiac cells are susceptible to weight gain through elevating oxidative stress (8). The discrepancy between the findings in this study and other studies can be due to differences in the assessment method (Colorimetric vs. ELISA or spectrophotometric) and diet type (Fat content in diet). It seems that oxidative stress is caused by diets of more than eight weeks that provide at least 50% of calories from fat (9,10,22). The HFD applied in this study provided only 40% of calories from fat.
According to the results of the current study, the cardiac SOD, GPX, and TAC levels increased significantly after eight weeks of RT. The effects of resistance training on cardiac oxidative stress biomarkers have received less attention than aerobic training. Consistent with the findings of the current study, Effting et al. (2019) reported that eight weeks of RT improved antioxidant systems in the cardiac tissue of rats fed with an HFD (8). Similarly, after eight weeks of RT in rats with high blood pressure, an increase in the antioxidant enzymes such as GPX and SOD was reported (16). Moreover, it has been reported that RT increased antioxidant capacity in the skeletal muscle of infarcted rats (23,24). The similarity of the results is likely due to using the same training protocols.
Regular exercise training increases the presence of mitochondrial uncoupling protein 2 (UCP2) in the mitochondrial membrane of the heart. This reduces electron leakage and decreases the formation of free radicals (25). Also, exercise increases the gene expression of antioxidant enzymes, which increases the antioxidant capacity and reduces oxidative stress due to the neutralization of free radicals (26). SOD is the first antioxidant enzyme in the body against free radicals and eliminates superoxide anions generated by NADPH oxidase to form oxygen and hydrogen peroxide. Therefore, GPX not only removes hydrogen peroxide but also prevents the production of other harmful free radicals such as hydroxyl radicals (27). Indeed, the GPX enzyme has more protective effects against oxidative damage than SOD, because the dismutation of superoxide anion by the SOD enzyme may increase hydrogen peroxide (12). Therefore, GPX is more effective in protecting cells, tissues, and organs against oxidative damage compared to SOD. In addition, GPX is present in cardiac tissue, especially cytosolic and mitochondrial parts (28). These pieces of evidence indicate that the GPX enzyme is of special importance as a defense mechanism in heart tissue. Based on the results obtained in the present study, RT protects against oxidative damage of cardiac tissue against free radicals by increasing SOD, GPX and TAC levels. RT seems to reduce ROS production in the mitochondrial respiratory chain by increasing muscle mass and changing energy substrates. As a result, the need to use antioxidant systems to suppress ROS is reduced, leading to an increase in antioxidant enzymes.
With the main objective of investigating the effects of RT on oxidative damage in the cardiac tissue induced by the HFD, we evaluated the levels of MDA, a byproduct of lipoperoxidation. We did not observe differences in this marker between groups. Gomes et al. (2020) also confirmed that 12 weeks of RT had no effect on the lipid hydroperoxide concentration (An oxidative stress biomarker) in the gastrocnemius muscle of rats with infarction (23). Moreover, 10 weeks of RT did not change the activity of antioxidant enzymes and lipid peroxidation markers of the liver tissue of ovariectomized rats (29). However, another study has reported a decreased MDA level in rat cardiac tissues following feeding HFD for 26 weeks (8). Moreover, after eight weeks of RT in hypertensive rats, a decrease in lipid peroxidation (Chemiluminescence) and protein oxidation of cardiac tissue was reported (16). It can be concluded that the stressor factors in this study were not enough to damage the cell membrane and increase MDA levels. Nevertheless, due to the difference in the methodology of the conducted research, other controlled studies with a similar design should be performed.
Conclusion
In the present study, cardiac SOD, GPX, and TAC levels were improved, thereby improving antioxidant defense, which may prevent lipid peroxidation and MDA accumulation in rats fed an HFD. RT used in this study modulated the oxidative stress in the cardiac tissue of rats fed with an HFD, thereby reducing obesity-related health complications. Therefore, performing RT is an effective non-pharmacological approach to reducing oxidative stress in the cardiac tissue. However, for more accurate conclusions, it is suggested that more studies be conducted in this field.
Acknowledgement
No acknowledgment.
Funding sources
This research received no external funding.
Ethical statement
All animal studies were performed following the standard ethical instructions for working with laboratory animals approved by the research ethics committee of the Sport Sciences Research Institute (Ethical code: IR.SSRI.REC.1401.1909).
Conflicts of interest
There is no conflict of interest.
Author contributions
All authors contributed to developing the protocol, data abstraction, and manuscript preparation.
Type of Article: Original article |
Subject:
General medicine
Received: 2024/01/6 | Accepted: 2024/03/20 | Published: 2024/03/30
Received: 2024/01/6 | Accepted: 2024/03/20 | Published: 2024/03/30
References
1. Kindel TL, Strande JL. Bariatric surgery as a treatment for heart failure: review of the literature and potential mechanisms. Surg Obes Relat Dis. 2018;14(1):117-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. 2019;11(11):2579. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Tapias V, Hu X, Luk KC, Sanders LH, Lee VM, Greenamyre JT. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci. 2017;74(15):2851-74. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9-10):1865-79. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Chen Y, Saari JT, Kang YJ. Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Radic Biol Med. 1994;17(6):529-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Adequate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev. 2014;19(1):15-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Esposito K, Ciotola M, Schisano B, Misso L, Giannetti G, Ceriello A, et al. Oxidative stress in the metabolic syndrome. J Endocrinol Invest. 2006;29(9):791-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Effting PS, Brescianini SMS, Sorato HR, Fernandes BB, Fidelis GDSP, Silva PRLD, et al. Resistance exercise modulates oxidative stress parameters and TNF-α content in the heart of mice with diet-induced obesity. Arq Bras Cardiol. 2019;112(5):545-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Emami SR, Jafari M, Haghshenas R, Ravasi A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J Exerc Nutrition Biochem. 2016;20(1):29-35. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Gujjala S, Putakala M, Bongu SBR, Ramaswamy R, Desireddy S. Preventive effect of Caralluma fimbriata against high-fat diet induced injury to heart by modulation of tissue lipids, oxidative stress and histological changes in Wistar rats. Arch Physiol Biochem. 2022;128(2):474-482. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, et al. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc. 2019;51(6):1270-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: Friend or foe? J Sport Health Sci. 2020;9(5):415-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Nocella C, Cammisotto V, Pigozzi F, Borrione P, Fossati C, D'Amico A, et al. Impairment between oxidant and antioxidant systems: short- and long-term implications for athletes' health. Nutrients. 2019;11(6):1353. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Thirupathi A, Wang M, Lin JK, Fekete G, István B, Baker JS, et al. Effect of different exercise modalities on oxidative stress: a systematic review. BioMed Res Int. 2021;2021:1947928. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. da Palma RK, Moraes-Silva IC, da Silva Dias D, Shimojo GL, Conti FF, Bernardes N, et al. Resistance or aerobic training decreases blood pressure and improves cardiovascular autonomic control and oxidative stress in hypertensive menopausal rats. J Appl Physiol (1985). 2016;121(4):1032-38. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Seo H, Lee NH, Ryu S. Antioxidant and antiapoptotic effects of pine needle powder ingestion and endurance training in high cholesterol-fed rats. J Exerc Nutrition Biochem. 2014;18(3):301-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2014;16(1):193-217. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Eslami Z, Mirghani SJ, Moghanlou AE, Norouzi A, Naseh H, Joshaghani H, et al. An efficient model of non-alcoholic fatty liver disease (NAFLD) versus current experimental models: effects of fructose, fat, and carbon tetrachloride on NAFLD. Hepat Mon. 2021;21(8):e117696. [View at Publisher] [DOI] [Google Scholar]
20. Zhang G, Wang X, Chung TY, Ye W, Hodge L, Zhang L, et al. Carbon tetrachloride (CCl4) accelerated development of non-alcoholic fatty liver disease (NAFLD)/steatohepatitis (NASH) in MS-NASH mice fed western diet supplemented with fructose (WDF). BMC Gastroenterol. 2020;20(1):339. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Philippe AG, Py G, Favier FB, Sanchez AM, Bonnieu A, Busso T, et al. Modeling the responses to resistance training in an animal experiment study. Biomed Res Int. 2015;2015:914860. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Skrzep-Poloczek B, Poloczek J, Chelmecka E, Dulska A, Romuk E, Idzik M, et al. The oxidative stress markers in the erythrocytes and heart muscle of obese rats: Relate to a high-fat diet but not to DJOS bariatric surgery. Antioxidants. 2020;9(2):183. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Gomes MJ, Pagan LU, Lima ARR, Reyes DRA, Martinez PF, Damatto FC, et al. Effects of aerobic and resistance exercise on cardiac remodelling and skeletal muscle oxidative stress of infarcted rats. J Cell Mol Med. 2020;24(9):5352-62. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Cai M, Wang Q, Liu Z, Jia D, Feng R, Tian Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018;213:40-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Bo H, Jiang N, Ma G, Qu J, Zhang G, Cao D, et al. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: Role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic Biol Med. 2008;44(7):1373-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Campos JC, Gomes KM, Ferreira JC. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol. 2013;62:107-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Tsutsui H, Kinugawa S, Matsushima S, Yokota T. Oxidative stress in cardiac and skeletal muscle dysfunction associated with diabetes mellitus. J Clin Biochem Nut. 2011;48(1):68-71. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Rodrigues MF, Stotzer US, Domingos MM, Deminice R, Shiguemoto GE, Tomaz LM, et al. Effects of ovariectomy and resistance training on oxidative stress markers in the rat liver. Clinics. 2013;68(9):1247-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






