Volume 12, Issue 1 (10-2024)
Jorjani Biomed J 2024, 12(1): 5-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khandashpour M, Rakhshaie M, Sharififar R, Livani S, Abdollahi N, Soleimannejad M, et al . Long-term pulmonary assessment in Iranian severe COVID-19 ICU survivors in 2020-2021: A Cohort Study. Jorjani Biomed J 2024; 12 (1) :5-9
URL: http://goums.ac.ir/jorjanijournal/article-1-1008-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1008-en.html
Long-term pulmonary assessment in Iranian severe COVID-19 ICU survivors in 2020-2021: A Cohort Study
Mahmoud Khandashpour1 

 , Mahtab Rakhshaie2
, Mahtab Rakhshaie2 

 , Rahmatollah Sharififar3
, Rahmatollah Sharififar3 

 , Somayeh Livani4
, Somayeh Livani4 

 , Nafiseh Abdollahi5
, Nafiseh Abdollahi5 

 , Mahdi Soleimannejad6
, Mahdi Soleimannejad6 

 , Fahimeh Abdollahi7
, Fahimeh Abdollahi7 

 , Mahila Monajati8
, Mahila Monajati8 




 , Mahtab Rakhshaie2
, Mahtab Rakhshaie2 

 , Rahmatollah Sharififar3
, Rahmatollah Sharififar3 

 , Somayeh Livani4
, Somayeh Livani4 

 , Nafiseh Abdollahi5
, Nafiseh Abdollahi5 

 , Mahdi Soleimannejad6
, Mahdi Soleimannejad6 

 , Fahimeh Abdollahi7
, Fahimeh Abdollahi7 

 , Mahila Monajati8
, Mahila Monajati8 


1- Department of Internal Medicine, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran ; Clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran
2- Clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran
3- Department of Infectious Diseases, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
4- Clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran; Department of Radiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
5- Department of Internal Medicine, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran; Golestan Rheumatology Research Center, Golestan University of Medical Sciences, Gorgan, Iran
6- Department of Radiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
7- Department of Internal Medicine, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
8- Golestan Rheumatology Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,mahilamonajati@gmail.com
2- Clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran
3- Department of Infectious Diseases, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
4- Clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran; Department of Radiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
5- Department of Internal Medicine, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran; Golestan Rheumatology Research Center, Golestan University of Medical Sciences, Gorgan, Iran
6- Department of Radiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
7- Department of Internal Medicine, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
8- Golestan Rheumatology Research Center, Golestan University of Medical Sciences, Gorgan, Iran ,
Full-Text [PDF 564 kb]
(2318 Downloads)
| Abstract (HTML) (8175 Views)
Full-Text: (1420 Views)
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused significant morbidity and mortality worldwide. As the number of COVID-19 pneumonia survivors continues to increase, there is growing concern about the long-term effects of the disease, including persistent abnormalities in lung imaging. Post-infectious pulmonary imaging, particularly chest CT scans, is often necessary to assess critical pulmonary pathologies during the recovery period. CT imaging provides valuable insights into structural lung abnormalities, complications, and disease progression. Numerous studies have utilized serial CT chest imaging to monitor the course of COVID-19 pneumonia, from the initial phase of infection to hospitalization and post-discharge (1).
Assessing the severity of lung involvement and predicting outcomes in COVID-19 is crucial for proper patient triage and resource allocation. CT scans have identified five patterns of lung parenchymal involvement that correlate with disease severity: no involvement, bronchopneumonia, consolidating pneumonia, progressive consolidating pneumonia, and diffuse alveolar damage patterns. The presence of fibroproliferative processes, such as crazy paving, bronchial dilatation, bronchiectasis, and diffuse alveolar damage, can serve as independent predictors of poor outcomes (2,3). Studies have shown that radiological resolution occurs gradually after discharge, with complete resolution rates of 53% after three weeks for ground-glass opacities (GGO) and fibrotic bands (4).
Comparisons between severe and non-severe COVID-19 patients have revealed a higher rate of fibrotic-like findings in the severe group. Patients with severe or critical disease experience more severe pulmonary complications, and their recovery is slower (5). A study by Yu et al. demonstrated that the risk of developing fibrosis is higher in patients with more severe clinical conditions, particularly those with elevated inflammatory indices (6).
Currently, there is insufficient information about long-term severe COVID-19 outcomes focused on Iranian patients. Given the potential for persistent respiratory complications and their association with disease severity and pulmonary fibrosis, it is crucial to address the considerable morbidity in this population, particularly among the critically ill. This study aimed to evaluate residual pulmonary involvement and pulmonary function in survivors of ICU-admitted severe COVID-19 patients one year after hospital discharge.
Methods
2.1 Study design
This cohort study, conducted in 2021 at Sayad Shirazi Hospital, a referral educational and medical center in Gorgan, northeastern Iran, included a comprehensive review of patients with severe COVID-19 pulmonary involvement who were hospitalized in the ICU between April 2020 and March 2021. COVID-19 was confirmed through PCR molecular testing with nasopharyngeal samples. Severe COVID-19 infection was defined based on the following signs and symptoms: shortness of breath, respiratory rate ≥30/min, blood oxygen saturation (SpO2) ≤93%, and lung involvement greater than 60%. The rigorous inclusion and exclusion criteria ensured the study's focus on severe COVID-19 cases, providing a comprehensive understanding of the long-term pulmonary effects.
Exclusion criteria encompassed patients with pre-existing chronic lung structural conditions such as asthma, chronic obstructive pulmonary disease (COPD), chronic bronchiectasis, fibrosis, lung cancer, lung metastasis, heart failure (Ejection fraction less than 40%), those who died after discharge, and pregnant individuals. These criteria were chosen to ensure that the study focused on the long-term pulmonary effects of severe COVID-19 in patients without pre-existing lung conditions or other significant health issues. This focus allows for a clearer understanding of the specific impact of severe COVID-19 on the lungs and the potential for recovery.
Sample size determination in this study took a pragmatic approach due to the challenges of recruiting patients during the COVID-19 outbreaks. All survived ICU admissions with COVID-19 (346 patients) were comprehensively screened, and patients were excluded based on predefined criteria until a cohort size of 30 was reached.
2.2 Data collection and patient recruitment
Medical records and lung CT scans of eligible patients were reviewed for data collection. The extracted information from patient files included baseline characteristics, past medical histories, clinical symptoms of COVID-19 infection, laboratory findings at admission (e.g., LDH, CRP, albumin), medications administered (Antiviral, corticosteroids, intravenous immunoglobulin), length of hospital and ICU stay, need for supplemental oxygen (Invasive and non-invasive), and readmission.
Patients were contacted via phone, and the study procedures were explained to them. Individuals were asked about the presence of any COVID-19-related symptoms, and if they expressed willingness to participate, a date was scheduled for chest CT scans and spirometry. Participants were provided with hard-copy reports of their spirometry and chest CT scan results and referred to a pulmonologist for further evaluation if necessary.
2.3 Radiological assessment and scoring
Two expert radiologists evaluated the initial and follow-up lung CT scans. The severity and pattern of lung involvement were assessed based on the scoring method outlined in the guidelines for the diagnosis and treatment of COVID-19 provided by Iran's Ministry of Health.
Severity scoring was based on the involvement of each lung lobe, with a score assigned to each of the six lobes. Scoring of lung involvement in lung CT scans was as follows: no involvement (0 points), less than 25% (1 point), 26-50% (2 points), 51-75% (3 points), and more than 76% (4 points). The total scores for all lobes were summed, with a score of up to 8 indicating moderate involvement and a score higher than 8 indicating severe involvement. The pattern of participation was categorized as GGO, consolidation, or nodules.
2.4 Pulmonary function testing
PFTs were performed for each patient, including forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the FEV1/FVC ratio. Spirometry was conducted using a Spirolab device. A healthcare technician administered the spirometry tests under the supervision of a pulmonologist, who interpreted the results following the guidelines set by the American Thoracic Society (ATS) (7-9). Pre-bronchodilator spirometry was performed initially, and then 400 mcg of salbutamol was administered for reversibility testing. After a 15-minute wait, post-bronchodilator spirometry was conducted. Spirometry results were interpreted based on four basic patterns: normal, obstructive, restrictive, or mixed.
In the normal pattern, both FEV1 and FVC were above 80% of predicted values, with an FEV1/FVC ratio above 0.7. The obstructive pattern w:as char:acterized by FEV1 below 80% of predicted values, with FVC either normal or reduced (Usually to a lesser degree than FEV1) and an FEV1/FVC ratio below 0.7. The restrictive pattern showed a normal or mildly reduced FEV1, FVC below 80% of predicted values, and an FEV1/FVC ratio above 0.7. It should be noted that restrictive lung disease cannot be definitively diagnosed based on spirometry alone. The mixed pattern exhibited reduced FEV1, reduced FVC, and a reduced FEV1/FVC ratio. Significant improvement in FEV1 after bronchodilator administration, with a change of greater than 12% and greater than 200 mL, was considered indicative of reversibility.
2.5 Data analysis
Data analysis was performed using SPSS version 18 software. Quantitative variables were presented as mean (Standard deviation), while categorical variables were expressed as numbers (Percentages). Pearson's correlation analysis was employed to explore the association between disease severity indicators during initial hospitalization, pulmonary function test results, and one-year follow-up lung CT scan findings. The significance level was set at 0.05.
Results
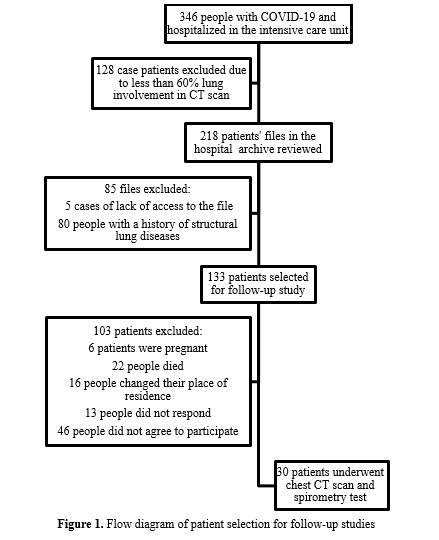
Thirty individuals underwent the follow-up assays, as depicted in Figure 1, which illustrates the patient enrollment process. The mean interval between hospital discharge and follow-up was 15 (2.5) months. The study population's mean age was 50 (13.5) years (Range: 30-76 years). The most commonly reported symptoms of COVID-19 were shortness of breath (93%), cough (87%), and fever (70%), with symptom onset occurring approximately six days prior to hospital admission.
Regarding the medications received, corticosteroids were administered to all patients (Dexamethasone 97%, methylprednisolone 73%, prednisolone 47%, and hydrocortisone 17%), while 90% received interferon alpha, and 60% received remdesivir. The typical regimen for intravenous corticosteroid administration consisted of methylprednisolone at a dose of 125 or 250 mg twice daily for three days, followed by dexamethasone at a dose of 8 mg three times to twice daily, and then a switch to oral prednisolone until discontinuation.
At the beginning of hospitalization, all patients required supplemental oxygen therapy, with six of them (20%) necessitating non-invasive ventilation.
3.1 Spiral chest CT scans during COVID-19 infection
Spiral chest CT scans at the time of COVID-19 infection revealed more than 60% parenchymal involvement in all patients. The predominant patterns of lung involvement observed were GGO in all patients (In all six lobes) and consolidation in 88% (Present in more than 85% of all lobes, predominantly in the right lower lobe and left lower lobe). The location of involvement was both peripheral and central in 83% of cases and peripheral only in 17%. No evidence of fibrosis was found, but three instances of pneumomediastinum were identified.
3.2 One-year follow-up spiral chest CT scan
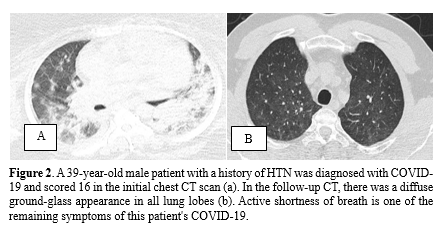
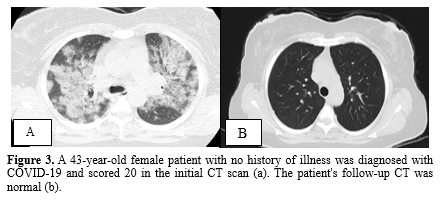
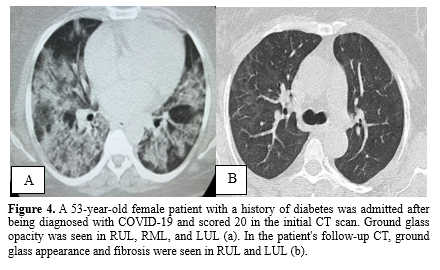
Mild fibrosis was observed in 21 individuals (70%) on the follow-up CT scans, while the remaining patients exhibited normal chest CT findings. The most common abnormalities observed were a fibrotic band (67%) and a fibrotic band with GGO (33%). No cases of pneumomediastinum or bronchiectasis were detected in any of the follow-up CT scans. Figures 2, 3, and 4 provide a visual comparison of baseline and follow-up chest CT scans for three patients.
3.3 One-year PFT
One-year follow-up spirometry revealed that 22 patients (73%) had normal spirometry results, six patients (20%) showed mild obstruction, and two patients (7%) exhibited hyper-reactive airway patterns. The mean change in forced expiratory volume in one second (FEV1) 15 minutes after salbutamol inhalation was 4.5 (15%) liters (Range: -23% to 43%). In two cases of mild obstruction and two patients with hyper-reactive spirometry, results showed positive reversibility testing with an FEV1 change of more than 12%. The mean percentage of post-bronchodilator FEV1/ FVC ratio was 86.5 (9%) (Range: 53-100%).
3.4 Baseline patients' characteristics and long-term outcomes
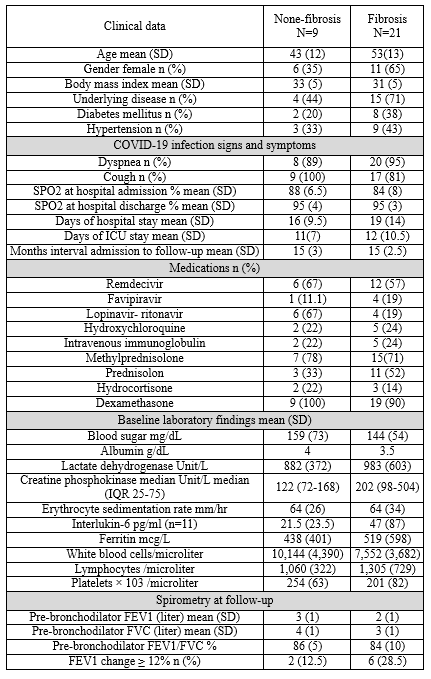
Table 1 demonstrates the baseline clinical status and follow-up spirometry in two subgroups of patients (Fibrosis and non-fibrosis). These two groups did not differ significantly in terms of demographic characteristics and inflammation indicators. Pearson correlation analysis revealed a significant mild correlation between the presence of fibrosis and lower serum albumin levels (r = -0.466, p = 0.05), older age (r = 0.351, p = 0.057), and lower platelet count (r = -0.311, p = 0.094).
3.5 Residual COVID-19 symptoms
Examination of residual pulmonary symptoms in these patients revealed that 60.7% (17 individuals) experienced shortness of breath, 21.4% (6 individuals) had no pulmonary symptoms, 10.7% (3 individuals) had a cough, and 7.1% (2 individuals) reported chest pain.
Discussion
This study investigated long-term PFT and chest CT scans of thirty ICU patients with severe COVID-19 infection after hospital discharge. The most common initial COVID-19 lung involvement types were GGO, consolidation, and mild bronchiectasis. At an average 15-month follow-up, chest CT scans predominantly showed abnormalities such as fibrotic bands and fibrotic bands with GGO. Additionally, spirometry results indicated that most patients had normal tests, with a small proportion exhibiting mild obstruction.
Previous studies have indicated that pulmonary fibrosis can develop early after COVID-19, with up to 39% of patients showing fibrosis after hospital discharge. However, the number of follow-up studies on critically ill COVID-19 patients is limited. One of the initial reports in 2020 focused on three critically ill COVID-19 patients (10) with severe acute respiratory distress syndrome (ARDS) who underwent follow-up PFT and radiological assessments within three months after discharge. The younger case showed improvement without abnormalities on chest CT and lung function tests. In comparison, the older case had residual radiological changes and impaired lung function during the follow-up period.
Certain risk factors are more closely associated with developing fibrosis after severe COVID-19. Older age is considered a major factor in some studies, alongside longer hospital stays, higher ICU admission rates, elevated C-reactive protein levels, lower albumin levels, ARDS, non-invasive mechanical ventilation, and higher chest CT scores (11-13). The study showed similar results, indicating that pulmonary fibrosis was more prevalent in older patients and those with lower serum albumin. High scores of crazy paving or consolidation and ICU admission are independent risk factors for fibrosis after COVID-19 (14).
High rates of chest CT abnormalities are observed when assessed shortly after COVID-19 infection. For instance, three months after discharge, more than 80% of patients exhibited abnormalities in chest CT, including fibrotic bands and GGO. Additionally, 40.6% of survivors still displayed pulmonary function abnormalities, which did not differ based on disease severity during hospitalization (15,16). Furthermore, lung diffusing capacity for carbon monoxide less than 80% could persist for 45 days (17). Another study of chest CT scans in severe COVID-19 patients over a longer period (More than a year) also showed the persistence of fibrotic interstitial lung abnormalities (18). Three serial chest CT scans up to one year after symptom onset showed that baseline abnormalities resolved in 61% of participants at three months and 75% at 12 months (19). The study revealed somewhat different results, indicating that severe COVID-19 pulmonary involvement completely resolved in fewer patients (30%), while mild fibrosis was present in two-thirds of survivors.
COVID-19-related symptoms may persist in half of the patients. In the study, more than 50% of patients still reported shortness of breath, although their spirometry results were nearly normal. While one study reported good functional recovery in the majority of critically ill patients with COVID-19-related ARDS after one year, with more than 80% reporting no dyspnea at rest and no difficulties performing usual activities, fibrotic-like changes were observed in 11% of patients based on one-year chest CT scans (20).
In a longer cohort study (Eighteen months after discharge), improvements in ventilated parenchyma and fibrotic-like alterations were reported (21). Chest CT scans and spirometry data showed a significantly increased volume of normal ventilated parenchyma. The values of GGOs and consolidations tended to decrease. There was no correlation between fibrotic-like changes and the treatment received, and the CT lung score did not correlate with spirometry results.
Spirometry is the most frequently performed pulmonary function test and is recommended for patients recovering from COVID-19 with persistent or evolving respiratory complications. Guidelines suggest that all patients recovering from severe pneumonia should undergo spirometry (22). It is considered a valuable health biomarker as it can identify individuals at risk of developing non-contagious diseases and premature mortality (23).
In some COVID-19 survivors, restrictive ventilatory defects and minor functional dysfunction may persist even after discharge, and these are not necessarily associated with disease severity (15). A study assessing pulmonary function at three months after discharge in severe COVID-19 cases found no significant differences in pre-bronchodilator FEV1 levels, pre-bronchodilator FVC levels, and FEV1/FVC% between non-severe and severe groups (16). The results of the study confirm that most patients could have normal PFT in the long term.
Most COVID-19 follow-up studies do not provide details about the medications used during hospitalization. However, higher rates of steroid use and elevated levels of D-dimer and LDH at discharge are noted among patients with fibrotic changes. Logistic regression analyses identified age, steroid therapy, bronchiectasis on chest CT at discharge, and opacity score at discharge as independent risk factors for developing pulmonary fibrosis seven months after discharge (12,14,24). The high rate of corticosteroid use in the study population might be attributed to the severity of pulmonary involvement, lower oxygen saturation, higher respiratory rate, and elevated C-reactive protein levels in these patients. Corticosteroids have been an important therapeutic option when anti-inflammatory or immunosuppressive effects are needed. COVID-19 is associated with a cytokine profile characterized by the activation of multiple pathways, including interleukins (25,26).
It is important to note that a high mortality rate of 16.5% (22 patients) was observed among the patients selected for follow-up investigation, occurring shortly after hospital discharge.
These findings underscore the importance of the primary hospital care team in identifying patients at higher risk for long-term complications and ensuring regular post-discharge monitoring.
The major limitation of the study was the difficulty of recalling patients for follow-up due to the ongoing COVID-19 outbreaks and patients' fear of visiting healthcare centers. This made it challenging to gather a larger sample size, potentially leading to selection bias and limited representation of the overall population.
Conclusion
Persistent respiratory complications following severe COVID-19 can lead to significant morbidity, especially among critically ill patients. The study found that most survivors of severe COVID-19 infection showed considerable improvement in chest CT findings and PFT at one-year follow-up. No significant differences were observed in the severity of primary lung involvement, medications used, and follow-up chest CT findings. The absence of a link offers comfort and trust in handling critical COVID-19 situations. It's crucial to thoroughly assess and monitor the respiratory health of those who've survived COVID-19 to ensure their best long-term recovery and to offer necessary support. We anticipate that these results will enhance confidence among healthcare professionals and offer peace of mind to patients and their families.
Acknowledgement
We sincerely thank all patients and their families. We appreciate the assistance of spirometry unit technicians of Deziani Specialized Clinic, Golestan University of Medical Sciences, Gorgan.
Funding sources
The study received financial support from the Research Vice Chancellor of Golestan University of Medical Sciences.
Ethical statement
Informed consent was obtained from all individuals included in the study, and the study protocol was approved by the Ethics Committee of Golestan University of Medical Sciences (Approval ID: IR.GOUMS.REC.1400.304).
Conflicts of interest
The author declares no conflict of interest, financial or otherwise.
Author contributions
Study concept and design: M.Kh, N. A, R. Sh, and M. M; analysis and interpretation of data: M. Kh, M. M, S. L, and M. S; drafting of the manuscript: M. R and M. M.; critical revision of the manuscript for important intellectual content: M.Kh and F. A.; statistical analysis: M. R and M.M.
The coronavirus disease 2019 (COVID-19) pandemic has caused significant morbidity and mortality worldwide. As the number of COVID-19 pneumonia survivors continues to increase, there is growing concern about the long-term effects of the disease, including persistent abnormalities in lung imaging. Post-infectious pulmonary imaging, particularly chest CT scans, is often necessary to assess critical pulmonary pathologies during the recovery period. CT imaging provides valuable insights into structural lung abnormalities, complications, and disease progression. Numerous studies have utilized serial CT chest imaging to monitor the course of COVID-19 pneumonia, from the initial phase of infection to hospitalization and post-discharge (1).
Assessing the severity of lung involvement and predicting outcomes in COVID-19 is crucial for proper patient triage and resource allocation. CT scans have identified five patterns of lung parenchymal involvement that correlate with disease severity: no involvement, bronchopneumonia, consolidating pneumonia, progressive consolidating pneumonia, and diffuse alveolar damage patterns. The presence of fibroproliferative processes, such as crazy paving, bronchial dilatation, bronchiectasis, and diffuse alveolar damage, can serve as independent predictors of poor outcomes (2,3). Studies have shown that radiological resolution occurs gradually after discharge, with complete resolution rates of 53% after three weeks for ground-glass opacities (GGO) and fibrotic bands (4).
Comparisons between severe and non-severe COVID-19 patients have revealed a higher rate of fibrotic-like findings in the severe group. Patients with severe or critical disease experience more severe pulmonary complications, and their recovery is slower (5). A study by Yu et al. demonstrated that the risk of developing fibrosis is higher in patients with more severe clinical conditions, particularly those with elevated inflammatory indices (6).
Currently, there is insufficient information about long-term severe COVID-19 outcomes focused on Iranian patients. Given the potential for persistent respiratory complications and their association with disease severity and pulmonary fibrosis, it is crucial to address the considerable morbidity in this population, particularly among the critically ill. This study aimed to evaluate residual pulmonary involvement and pulmonary function in survivors of ICU-admitted severe COVID-19 patients one year after hospital discharge.
Methods
2.1 Study design
This cohort study, conducted in 2021 at Sayad Shirazi Hospital, a referral educational and medical center in Gorgan, northeastern Iran, included a comprehensive review of patients with severe COVID-19 pulmonary involvement who were hospitalized in the ICU between April 2020 and March 2021. COVID-19 was confirmed through PCR molecular testing with nasopharyngeal samples. Severe COVID-19 infection was defined based on the following signs and symptoms: shortness of breath, respiratory rate ≥30/min, blood oxygen saturation (SpO2) ≤93%, and lung involvement greater than 60%. The rigorous inclusion and exclusion criteria ensured the study's focus on severe COVID-19 cases, providing a comprehensive understanding of the long-term pulmonary effects.
Exclusion criteria encompassed patients with pre-existing chronic lung structural conditions such as asthma, chronic obstructive pulmonary disease (COPD), chronic bronchiectasis, fibrosis, lung cancer, lung metastasis, heart failure (Ejection fraction less than 40%), those who died after discharge, and pregnant individuals. These criteria were chosen to ensure that the study focused on the long-term pulmonary effects of severe COVID-19 in patients without pre-existing lung conditions or other significant health issues. This focus allows for a clearer understanding of the specific impact of severe COVID-19 on the lungs and the potential for recovery.
Sample size determination in this study took a pragmatic approach due to the challenges of recruiting patients during the COVID-19 outbreaks. All survived ICU admissions with COVID-19 (346 patients) were comprehensively screened, and patients were excluded based on predefined criteria until a cohort size of 30 was reached.
2.2 Data collection and patient recruitment
Medical records and lung CT scans of eligible patients were reviewed for data collection. The extracted information from patient files included baseline characteristics, past medical histories, clinical symptoms of COVID-19 infection, laboratory findings at admission (e.g., LDH, CRP, albumin), medications administered (Antiviral, corticosteroids, intravenous immunoglobulin), length of hospital and ICU stay, need for supplemental oxygen (Invasive and non-invasive), and readmission.
Patients were contacted via phone, and the study procedures were explained to them. Individuals were asked about the presence of any COVID-19-related symptoms, and if they expressed willingness to participate, a date was scheduled for chest CT scans and spirometry. Participants were provided with hard-copy reports of their spirometry and chest CT scan results and referred to a pulmonologist for further evaluation if necessary.
2.3 Radiological assessment and scoring
Two expert radiologists evaluated the initial and follow-up lung CT scans. The severity and pattern of lung involvement were assessed based on the scoring method outlined in the guidelines for the diagnosis and treatment of COVID-19 provided by Iran's Ministry of Health.
Severity scoring was based on the involvement of each lung lobe, with a score assigned to each of the six lobes. Scoring of lung involvement in lung CT scans was as follows: no involvement (0 points), less than 25% (1 point), 26-50% (2 points), 51-75% (3 points), and more than 76% (4 points). The total scores for all lobes were summed, with a score of up to 8 indicating moderate involvement and a score higher than 8 indicating severe involvement. The pattern of participation was categorized as GGO, consolidation, or nodules.
2.4 Pulmonary function testing
PFTs were performed for each patient, including forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the FEV1/FVC ratio. Spirometry was conducted using a Spirolab device. A healthcare technician administered the spirometry tests under the supervision of a pulmonologist, who interpreted the results following the guidelines set by the American Thoracic Society (ATS) (7-9). Pre-bronchodilator spirometry was performed initially, and then 400 mcg of salbutamol was administered for reversibility testing. After a 15-minute wait, post-bronchodilator spirometry was conducted. Spirometry results were interpreted based on four basic patterns: normal, obstructive, restrictive, or mixed.
In the normal pattern, both FEV1 and FVC were above 80% of predicted values, with an FEV1/FVC ratio above 0.7. The obstructive pattern w:as char:acterized by FEV1 below 80% of predicted values, with FVC either normal or reduced (Usually to a lesser degree than FEV1) and an FEV1/FVC ratio below 0.7. The restrictive pattern showed a normal or mildly reduced FEV1, FVC below 80% of predicted values, and an FEV1/FVC ratio above 0.7. It should be noted that restrictive lung disease cannot be definitively diagnosed based on spirometry alone. The mixed pattern exhibited reduced FEV1, reduced FVC, and a reduced FEV1/FVC ratio. Significant improvement in FEV1 after bronchodilator administration, with a change of greater than 12% and greater than 200 mL, was considered indicative of reversibility.
2.5 Data analysis
Data analysis was performed using SPSS version 18 software. Quantitative variables were presented as mean (Standard deviation), while categorical variables were expressed as numbers (Percentages). Pearson's correlation analysis was employed to explore the association between disease severity indicators during initial hospitalization, pulmonary function test results, and one-year follow-up lung CT scan findings. The significance level was set at 0.05.
Results
Thirty individuals underwent the follow-up assays, as depicted in Figure 1, which illustrates the patient enrollment process. The mean interval between hospital discharge and follow-up was 15 (2.5) months. The study population's mean age was 50 (13.5) years (Range: 30-76 years). The most commonly reported symptoms of COVID-19 were shortness of breath (93%), cough (87%), and fever (70%), with symptom onset occurring approximately six days prior to hospital admission.
Regarding the medications received, corticosteroids were administered to all patients (Dexamethasone 97%, methylprednisolone 73%, prednisolone 47%, and hydrocortisone 17%), while 90% received interferon alpha, and 60% received remdesivir. The typical regimen for intravenous corticosteroid administration consisted of methylprednisolone at a dose of 125 or 250 mg twice daily for three days, followed by dexamethasone at a dose of 8 mg three times to twice daily, and then a switch to oral prednisolone until discontinuation.
At the beginning of hospitalization, all patients required supplemental oxygen therapy, with six of them (20%) necessitating non-invasive ventilation.

3.1 Spiral chest CT scans during COVID-19 infection
Spiral chest CT scans at the time of COVID-19 infection revealed more than 60% parenchymal involvement in all patients. The predominant patterns of lung involvement observed were GGO in all patients (In all six lobes) and consolidation in 88% (Present in more than 85% of all lobes, predominantly in the right lower lobe and left lower lobe). The location of involvement was both peripheral and central in 83% of cases and peripheral only in 17%. No evidence of fibrosis was found, but three instances of pneumomediastinum were identified.
3.2 One-year follow-up spiral chest CT scan
Mild fibrosis was observed in 21 individuals (70%) on the follow-up CT scans, while the remaining patients exhibited normal chest CT findings. The most common abnormalities observed were a fibrotic band (67%) and a fibrotic band with GGO (33%). No cases of pneumomediastinum or bronchiectasis were detected in any of the follow-up CT scans. Figures 2, 3, and 4 provide a visual comparison of baseline and follow-up chest CT scans for three patients.



3.3 One-year PFT
One-year follow-up spirometry revealed that 22 patients (73%) had normal spirometry results, six patients (20%) showed mild obstruction, and two patients (7%) exhibited hyper-reactive airway patterns. The mean change in forced expiratory volume in one second (FEV1) 15 minutes after salbutamol inhalation was 4.5 (15%) liters (Range: -23% to 43%). In two cases of mild obstruction and two patients with hyper-reactive spirometry, results showed positive reversibility testing with an FEV1 change of more than 12%. The mean percentage of post-bronchodilator FEV1/ FVC ratio was 86.5 (9%) (Range: 53-100%).
3.4 Baseline patients' characteristics and long-term outcomes
Table 1 demonstrates the baseline clinical status and follow-up spirometry in two subgroups of patients (Fibrosis and non-fibrosis). These two groups did not differ significantly in terms of demographic characteristics and inflammation indicators. Pearson correlation analysis revealed a significant mild correlation between the presence of fibrosis and lower serum albumin levels (r = -0.466, p = 0.05), older age (r = 0.351, p = 0.057), and lower platelet count (r = -0.311, p = 0.094).
|
Table 1. Comparison of baseline patients’ clinical status and follow-up studies
 |
Examination of residual pulmonary symptoms in these patients revealed that 60.7% (17 individuals) experienced shortness of breath, 21.4% (6 individuals) had no pulmonary symptoms, 10.7% (3 individuals) had a cough, and 7.1% (2 individuals) reported chest pain.
Discussion
This study investigated long-term PFT and chest CT scans of thirty ICU patients with severe COVID-19 infection after hospital discharge. The most common initial COVID-19 lung involvement types were GGO, consolidation, and mild bronchiectasis. At an average 15-month follow-up, chest CT scans predominantly showed abnormalities such as fibrotic bands and fibrotic bands with GGO. Additionally, spirometry results indicated that most patients had normal tests, with a small proportion exhibiting mild obstruction.
Previous studies have indicated that pulmonary fibrosis can develop early after COVID-19, with up to 39% of patients showing fibrosis after hospital discharge. However, the number of follow-up studies on critically ill COVID-19 patients is limited. One of the initial reports in 2020 focused on three critically ill COVID-19 patients (10) with severe acute respiratory distress syndrome (ARDS) who underwent follow-up PFT and radiological assessments within three months after discharge. The younger case showed improvement without abnormalities on chest CT and lung function tests. In comparison, the older case had residual radiological changes and impaired lung function during the follow-up period.
Certain risk factors are more closely associated with developing fibrosis after severe COVID-19. Older age is considered a major factor in some studies, alongside longer hospital stays, higher ICU admission rates, elevated C-reactive protein levels, lower albumin levels, ARDS, non-invasive mechanical ventilation, and higher chest CT scores (11-13). The study showed similar results, indicating that pulmonary fibrosis was more prevalent in older patients and those with lower serum albumin. High scores of crazy paving or consolidation and ICU admission are independent risk factors for fibrosis after COVID-19 (14).
High rates of chest CT abnormalities are observed when assessed shortly after COVID-19 infection. For instance, three months after discharge, more than 80% of patients exhibited abnormalities in chest CT, including fibrotic bands and GGO. Additionally, 40.6% of survivors still displayed pulmonary function abnormalities, which did not differ based on disease severity during hospitalization (15,16). Furthermore, lung diffusing capacity for carbon monoxide less than 80% could persist for 45 days (17). Another study of chest CT scans in severe COVID-19 patients over a longer period (More than a year) also showed the persistence of fibrotic interstitial lung abnormalities (18). Three serial chest CT scans up to one year after symptom onset showed that baseline abnormalities resolved in 61% of participants at three months and 75% at 12 months (19). The study revealed somewhat different results, indicating that severe COVID-19 pulmonary involvement completely resolved in fewer patients (30%), while mild fibrosis was present in two-thirds of survivors.
COVID-19-related symptoms may persist in half of the patients. In the study, more than 50% of patients still reported shortness of breath, although their spirometry results were nearly normal. While one study reported good functional recovery in the majority of critically ill patients with COVID-19-related ARDS after one year, with more than 80% reporting no dyspnea at rest and no difficulties performing usual activities, fibrotic-like changes were observed in 11% of patients based on one-year chest CT scans (20).
In a longer cohort study (Eighteen months after discharge), improvements in ventilated parenchyma and fibrotic-like alterations were reported (21). Chest CT scans and spirometry data showed a significantly increased volume of normal ventilated parenchyma. The values of GGOs and consolidations tended to decrease. There was no correlation between fibrotic-like changes and the treatment received, and the CT lung score did not correlate with spirometry results.
Spirometry is the most frequently performed pulmonary function test and is recommended for patients recovering from COVID-19 with persistent or evolving respiratory complications. Guidelines suggest that all patients recovering from severe pneumonia should undergo spirometry (22). It is considered a valuable health biomarker as it can identify individuals at risk of developing non-contagious diseases and premature mortality (23).
In some COVID-19 survivors, restrictive ventilatory defects and minor functional dysfunction may persist even after discharge, and these are not necessarily associated with disease severity (15). A study assessing pulmonary function at three months after discharge in severe COVID-19 cases found no significant differences in pre-bronchodilator FEV1 levels, pre-bronchodilator FVC levels, and FEV1/FVC% between non-severe and severe groups (16). The results of the study confirm that most patients could have normal PFT in the long term.
Most COVID-19 follow-up studies do not provide details about the medications used during hospitalization. However, higher rates of steroid use and elevated levels of D-dimer and LDH at discharge are noted among patients with fibrotic changes. Logistic regression analyses identified age, steroid therapy, bronchiectasis on chest CT at discharge, and opacity score at discharge as independent risk factors for developing pulmonary fibrosis seven months after discharge (12,14,24). The high rate of corticosteroid use in the study population might be attributed to the severity of pulmonary involvement, lower oxygen saturation, higher respiratory rate, and elevated C-reactive protein levels in these patients. Corticosteroids have been an important therapeutic option when anti-inflammatory or immunosuppressive effects are needed. COVID-19 is associated with a cytokine profile characterized by the activation of multiple pathways, including interleukins (25,26).
It is important to note that a high mortality rate of 16.5% (22 patients) was observed among the patients selected for follow-up investigation, occurring shortly after hospital discharge.
These findings underscore the importance of the primary hospital care team in identifying patients at higher risk for long-term complications and ensuring regular post-discharge monitoring.
The major limitation of the study was the difficulty of recalling patients for follow-up due to the ongoing COVID-19 outbreaks and patients' fear of visiting healthcare centers. This made it challenging to gather a larger sample size, potentially leading to selection bias and limited representation of the overall population.
Conclusion
Persistent respiratory complications following severe COVID-19 can lead to significant morbidity, especially among critically ill patients. The study found that most survivors of severe COVID-19 infection showed considerable improvement in chest CT findings and PFT at one-year follow-up. No significant differences were observed in the severity of primary lung involvement, medications used, and follow-up chest CT findings. The absence of a link offers comfort and trust in handling critical COVID-19 situations. It's crucial to thoroughly assess and monitor the respiratory health of those who've survived COVID-19 to ensure their best long-term recovery and to offer necessary support. We anticipate that these results will enhance confidence among healthcare professionals and offer peace of mind to patients and their families.
Acknowledgement
We sincerely thank all patients and their families. We appreciate the assistance of spirometry unit technicians of Deziani Specialized Clinic, Golestan University of Medical Sciences, Gorgan.
Funding sources
The study received financial support from the Research Vice Chancellor of Golestan University of Medical Sciences.
Ethical statement
Informed consent was obtained from all individuals included in the study, and the study protocol was approved by the Ethics Committee of Golestan University of Medical Sciences (Approval ID: IR.GOUMS.REC.1400.304).
Conflicts of interest
The author declares no conflict of interest, financial or otherwise.
Author contributions
Study concept and design: M.Kh, N. A, R. Sh, and M. M; analysis and interpretation of data: M. Kh, M. M, S. L, and M. S; drafting of the manuscript: M. R and M. M.; critical revision of the manuscript for important intellectual content: M.Kh and F. A.; statistical analysis: M. R and M.M.
Type of Article: Original article |
Subject:
General medicine
Received: 2023/12/28 | Accepted: 2024/01/10 | Published: 2024/01/31
Received: 2023/12/28 | Accepted: 2024/01/10 | Published: 2024/01/31
References
1. Chohan A, Choudhury S, Dadhwal R, Vakil AP, Franco R, Taweesedt PT. Follow-up computed tomography scan in post-COVID-19 pneumonia. World J Radiol. 2022;14(4):104-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Jin C, Tian C, Wang Y, Wu CC, Zhao H, Liang T, et al. A pattern categorization of CT findings to predict outcome of COVID-19 pneumonia. Front Public Health. 2020;8:567672. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Pugliese L, Sbordone FP, Grimaldi F, Ricci F, Di Tosto F, Spiritigliozzi L, et al. Chest computed tomography scoring in patients with novel coronavirus-infected pneumonia: correlation with clinical and laboratory features and disease outcome. In Vivo. 2020;34(6):3735-46. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Liu D, Zhang W, Pan F, Li L, Yang L, Zheng D, et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21(1):125. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Zhang D, Zhang C, Li X, Zhao J, An C, Peng C, et al. Thin-section computed tomography findings and longitudinal variations of the residual pulmonary sequelae after discharge in patients with COVID-19: a short-term follow-up study. Eur Radiol. 2021;31(9):7172-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746-55. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70-88. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463-72. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Zha L, Shen Y, Pan L, Han M, Yang G, Teng X, et al. Follow-up study on pulmonary function and radiological changes in critically ill patients with COVID-19. J Infect. 2021;82(1):159-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Wei J, Yang H, Lei P, Fan B, Qiu Y, Zeng B, et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28(3):383-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Zhao Y-m, Shang Y-m, Song W-b, Li Q-q, Xie H, Xu Q-f, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Yasin R, Gomaa AAK, Ghazy T, Hassanein SA, Ibrahem RAL, Khalifa MH. Predicting lung fibrosis in post-COVID-19 patients after discharge with follow-up chest CT findings. Egypt J Radiol Nucl Med. 2021;52(1):118. [View at Publisher] [DOI] [Google Scholar]
15. You J, Zhang L, Zhang J, Hu F, Chen L, Dong Y, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020;81(2):e150-2. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Liao X, Wang Y, He Z, Yun Y, Hu M, Ma Z, et al. Three-month pulmonary function and radiological outcomes in COVID-19 survivors: a longitudinal patient cohort study. Open Forum Infect Dis; 2020;8(9):ofaa540. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Blanco JR, Cobos-Ceballos MJ, Navarro F, Sanjoaquin I, de Las Revillas FA, Bernal E, et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin Microbiol Infect. 2021;27(6):892-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Han X, Fan Y, Alwalid O, Zhang X, Jia X, Zheng Y, et al. Fibrotic interstitial lung abnormalities at 1-year follow-up CT after severe COVID-19. Radiology. 2021;301(3):E438-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Pan F, Yang L, Liang B, Ye T, Li L, Li L, et al. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19. Radiology. 2022;302(3):709-19. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Zangrillo A, Belletti A, Palumbo D, Calvi MR, Guzzo F, Fominskiy EV, et al. One-year multidisciplinary follow-up of patients with COVID-19 requiring invasive mechanical ventilation. J Cardiothorac Vasc Anesth. 2022;36(5):1354-63. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Barini M, Percivale I, Danna P, Longo V, Costantini P, Paladini A, et al. 18 months computed tomography follow-up after Covid-19 interstitial pneumonia. J Public Health Res. 2022;11(2):2782. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Crimi C, Impellizzeri P, Campisi R, Nolasco S, Spanevello A, Crimi N. Practical considerations for spirometry during the COVID-19 outbreak: Literature review and insights. Pulmonology. 2021;27(5):438-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Townsend MC. Spirometry in occupational health-2020. J Occup Environ Med. 2020;62(5):e208-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Liu M, Lv F, Huang Y, Xiao K. Follow-Up Study of the Chest CT Characteristics of COVID-19 Survivors Seven Months After Recovery. Front Med. 2021;8:1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Papamanoli A, Yoo J, Grewal P, Predun W, Hotelling J, Jacob R, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Invest. 2021;51(2):e13458. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



