Volume 11, Issue 4 (12-2023)
Jorjani Biomed J 2023, 11(4): 8-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Heidarzadeh S, Ashrafmansouri S. Molecular docking study of cytochalasin H and Fascin interactions as prospective targets for gastric cancer. Jorjani Biomed J 2023; 11 (4) :8-10
URL: http://goums.ac.ir/jorjanijournal/article-1-1002-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1002-en.html
1- Department of Biology, Faculty of Science, University of Zabol, Zabol, Iran
2- Department of Biology, School of Science, Shiraz University, Shiraz, Iran ,s.ashrafmansouri@shirazu.ac.ir
2- Department of Biology, School of Science, Shiraz University, Shiraz, Iran ,
Keywords: Cytochalasin H, Drug design, Fascin protein, Molecular docking simulation, Stomach neoplasms
Full-Text [PDF 453 kb]
(2529 Downloads)
| Abstract (HTML) (8083 Views)
Full-Text: (1614 Views)
Introduction
Gastric cancer is a prevalent and aggressive form of cancer that accounts for 8.3% of cancer-related deaths and affects individuals worldwide, with a particularly high incidence among males (1,2). According to the International Classification of Diseases for Oncology (ICD-O), gastric cancer is identified by the specific code 8602/0, characterizing the disease based on its histological and morphological features (3). Gastric carcinoma is a complex disease influenced by various factors, including both environmental and genetic risk factors (4). One of the significant challenges in treating gastric cancer is its tendency to metastasize, which hinders successful treatment outcomes (5). Cell motility and adhesiveness play crucial roles in the aggressive nature of gastric cancer, as they cause the rearrangement of the cytoskeleton structure (6). The cytoskeleton, comprised of the microtubule and microfilament systems, mainly relies on actin-bundling proteins, such as cortactin and fascin (7). These proteins are instrumental in regulating the organization and stability of the cytoskeleton. Fascin comprises two chains and four B-trefoil domains with a length of 496 sequences. Recent studies have provided evidence that fascin protein acts as a metastasis promoter and plays a crucial role in regulating cell migration through cellular signaling pathways (8,9). Overexpression of this protein has been documented in various types of tumors, including breast, bladder, prostate, brain tumors, and esophageal carcinoma (10). In these cancers, fascin overexpression is associated with enhanced cell invasion and metastasis of tumor cells to surrounding tissues and has been identified as a prognostic marker in gastric cancer (11,12). In a detailed crystal structure of fascin, ligand molecules, such as glycerol and polyethylene glycol, are found to be bound within pockets in the two primary actin-binding sites. These molecules offer insights for the strategic development of novel anticancer fascin inhibitors.
In contrast, cytochalasin compounds affect the cytoskeleton by targeting microfilaments and microtubules, disrupting actin polymerization processes (13,14). Cytochalasins belong to a class of alkaloid mycotoxins and are commonly found in fungi, with extraction often performed from an endophytic fungus called Rhinocladiella sp. (15). Unlike conventional chemotherapy drugs, which often lead to severe side effects, drug resistance, and suboptimal long-term outcomes, secondary metabolites like cytochalasin H exhibit high biological activity and low toxicity. Cytochalasin H, as a metabolite of Phomopsis paspali, has shown significant effects on cytoskeleton reorganization and its anti-inflammatory, antifungal, and antitumor properties have been reported (16). In addition, its antitumor effects are achieved through distinct mechanisms, including the induction of apoptosis and the inhibition of angiogenesis (17,18,19). In the present study, we employed in silico analyses to explore potential interactions between cytochalasin H (inhibitor) and fascin. Blocking fascin binding sites is considered a vital target for antimetastatic drugs. Our study shows that cytochalasin H has the potential to inhibit cell migration as a secondary metabolite by blocking this protein. Molecular docking is a widely used method in drug discovery and cancer research to investigate the stable interaction between small molecules and proteins (20). This approach holds a valuable preclinical tool for the development of more effective and potent anticancer therapies (21).
Methods
The two-dimensional structure of cytochalasin H as a ligand was designed and optimized using the HyperChem 7.0 software (22) and the Polak-Ribiere algorithm to achieve the most stable structure. The ligand molecule in HIN format was converted to the Protein Data Bank (PDB) format using Open Babel 2.4.1 tool (22). This format is compatible with the PDB. The crystal structure of the human target protein fascin with a resolution of 2Å was obtained from the Brookhaven PDB (Code: 3p53). Discovery Studio software was used to remove water molecules and default ligands from the fascin protein structure. This step prepares the protein for further analysis. AutoDock Tools 1.5.6, part of the molecular graphics laboratory (MGL) software package, was used to convert the pre-processed fascin protein structure from PDB to PDB, Partial Charge (Q), and Atom Type (T) (PDBQT) format. PDBQT is the format required for AutoDock Vina, the docking software used in the study (23).
We performed rigid docking to predict atomic-level interaction between cytochalasin H and fascin protein by AutoDock Vina 1.1.2. (http://vina.scripps.edu). The results obtained from docking were analyzed using various software tools, such as LigPlot+ v.1.4.5, Discovery Studio 4.5, and PyMOL v.1.9. These tools provide visualization and analysis of the ligand-protein interactions, allowing for a more detailed understanding of the binding mode and potential binding sites.
Results
Cytochalasins have been found to impact various cellular functions, drug delivery, and chemotherapy. They can also induce a significant clinical response in cell systems as antitumor drugs (8). The molecular binding position between the ligand and protein was determined in the present study using a grid box. This function plays a crucial role in the selection and efficiency of molecular docking by restricting the search area in AutoDock. The coordinates of the grid box for the ligand binding region (actin-binding site) were set as 27.083 for X, -52.227 for Y, and 33.496 for Z. The size of the grid box was maintained at 42 * 34 * 40, with a grid spacing of 0.375 Å. The results obtained from AutoDock Vina 1.1.2 indicated that the affinity value for the interactions between fascin protein and cytochalasin H was determined to be -9.5 kcal/mol (Figure 1).

Cytochalasin H was docked to residues located in the active site of fascin protein. Analysis conducted using LigPlot+ v.1.4.5 software revealed the two-dimensional protein-ligand interactions, as shown in Figure 2. Amino acids present in the active site formed interactions with the ligand. The hydrophobic contacts involved Asp337, Val435, Thr434, Thr442, Gly436, Thr401, Thr403, Trp340, Cys456, Val485, Lys303, Ser444, and Lys304, which played a role in binding cytochalasin H to the fascin protein. Furthermore, three hydrogen bonds were observed with Arg348, Thr302, and Ile338. Among them, the hydrogen bond with Ile338 had a length of 2.93 Å and was related to a hydrophobic residue. Two hydrogen bonds, with lengths of 3.03 Å and 3.07 Å, were formed with the polar aliphatic residues Arg348 and Thr302, respectively, as depicted in Figure 2. The primary bonds responsible for stabilizing the ligand-enzyme complex were hydrogen bonds and hydrophobic interactions (24).

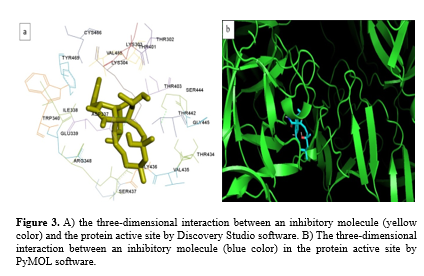
In this study, Discovery Studio 4.5 and PyMOL v.1.9 software was used for the three-dimensional analysis of molecular docking results. Figure 3a shows the ligand and protein interaction in the active site. Cytochalasin H is at its best energy level with the fascin protein. High-quality images were obtained from the ligand binding site to the protein. Figures 3a and 3b show an active site in the protein. This site is located at the interface between chain A and chain B.
Therefore, based on these results, cytochalasin H can be introduced as an effective antitumor agent in gastric cancer. However, further studies are needed to explore the exact mechanism.
Discussion
Given that gastric cancer is a multifactorial disease associated with high mortality rates, numerous studies have employed molecular docking to predict suitable drugs. In a study by Imani-Saber et al. in 2015, imatinib was proposed as a suitable ligand to inhibitcasein kinase 2 (CK2) and preserve promyelocytic leukemia (PML) as a tumor suppressor gene, focusing on gastric cancers caused by Helicobacter pylori (24). Also, much attention has been paid to fascin as a crucial actin-bundling protein (F-actin) pivotal in creating cell surface protrusions that facilitate cell migration (20,11). In normal adult epithelial tissues, fascin is typically absent or down-regulated. However, it is notably up-regulated in various carcinomas, demonstrating a positive correlation with invasion, metastasis, and unfavorable prognosis (11,25). Conversely, inhibiting fascin through an inhibitor can prevent the invasion of cancer cells. Multiple studies have investigated the effects of cytochalasins on different types of cancer or infectious diseases (26). The effects of cytochalasin H on apoptosis and migration were investigated in the human lung adenocarcinoma cell line A549. The treatment of A549 cells with cytochalasin H resulted in cell cycle arrest at the G2/M phase and showed strong anti-migratory activities (27). In our study, the main objective was to investigate and understand the underlying interactions through which cytochalasin H inhibits the migration ability of cancer cells, ultimately impeding the progression and metastasis of the disease. In line with this objective, we employed molecular docking techniques to investigate the potential of cytochalasin H as an inhibitor of fascin protein. These findings suggest that cytochalasin H may hold promise as a suitable inhibitor for targeting fascin. This study provides valuable insights into developing therapeutic strategies for gastric cancer treatment by elucidating the molecular interactions and binding energies. However, the precise determination of the inhibitor's concentration, dosage, and efficacy in inhibiting cancer cells requires additional in vitro and in vivo investigations.
Conclusion
In conclusion, targeting fascin represents a promising approach for controlling and treating gastric cancer. Inhibition of this protein probably can impact metastasis processes and cytoskeleton polymerization significantly. The docking results demonstrate that cytochalasin H has the potential to serve as a candidate drug for inhibiting and facilitating gastric cancer therapy. So, further investigations, including in vitro and in vivo studies, are warranted to validate these findings and explore the precise mechanism of action. The development of novel drug design strategies targeting fascin could pave the way for more effective treatments for gastric cancer, another cancer, or even the metastasis of other cancers by influencing the cellular skeleton.
Acknowledgement
Not applicable.
Funding sources
This study did not receive any funds.
Ethical statement
Not applicable. This article was conducted using software and data analysis.
Conflicts of interest
The authors declared no conflict of interest.
Author contributions
We confirm that all authors were involved in writing the article. S.H.: A master’s graduate, contribution in the manuscript conceptualization, writing, and introduction, and draft preparation; S.A.: A Ph.D. student, contribution in the manuscript methodology, software, writing, and results.
Gastric cancer is a prevalent and aggressive form of cancer that accounts for 8.3% of cancer-related deaths and affects individuals worldwide, with a particularly high incidence among males (1,2). According to the International Classification of Diseases for Oncology (ICD-O), gastric cancer is identified by the specific code 8602/0, characterizing the disease based on its histological and morphological features (3). Gastric carcinoma is a complex disease influenced by various factors, including both environmental and genetic risk factors (4). One of the significant challenges in treating gastric cancer is its tendency to metastasize, which hinders successful treatment outcomes (5). Cell motility and adhesiveness play crucial roles in the aggressive nature of gastric cancer, as they cause the rearrangement of the cytoskeleton structure (6). The cytoskeleton, comprised of the microtubule and microfilament systems, mainly relies on actin-bundling proteins, such as cortactin and fascin (7). These proteins are instrumental in regulating the organization and stability of the cytoskeleton. Fascin comprises two chains and four B-trefoil domains with a length of 496 sequences. Recent studies have provided evidence that fascin protein acts as a metastasis promoter and plays a crucial role in regulating cell migration through cellular signaling pathways (8,9). Overexpression of this protein has been documented in various types of tumors, including breast, bladder, prostate, brain tumors, and esophageal carcinoma (10). In these cancers, fascin overexpression is associated with enhanced cell invasion and metastasis of tumor cells to surrounding tissues and has been identified as a prognostic marker in gastric cancer (11,12). In a detailed crystal structure of fascin, ligand molecules, such as glycerol and polyethylene glycol, are found to be bound within pockets in the two primary actin-binding sites. These molecules offer insights for the strategic development of novel anticancer fascin inhibitors.
In contrast, cytochalasin compounds affect the cytoskeleton by targeting microfilaments and microtubules, disrupting actin polymerization processes (13,14). Cytochalasins belong to a class of alkaloid mycotoxins and are commonly found in fungi, with extraction often performed from an endophytic fungus called Rhinocladiella sp. (15). Unlike conventional chemotherapy drugs, which often lead to severe side effects, drug resistance, and suboptimal long-term outcomes, secondary metabolites like cytochalasin H exhibit high biological activity and low toxicity. Cytochalasin H, as a metabolite of Phomopsis paspali, has shown significant effects on cytoskeleton reorganization and its anti-inflammatory, antifungal, and antitumor properties have been reported (16). In addition, its antitumor effects are achieved through distinct mechanisms, including the induction of apoptosis and the inhibition of angiogenesis (17,18,19). In the present study, we employed in silico analyses to explore potential interactions between cytochalasin H (inhibitor) and fascin. Blocking fascin binding sites is considered a vital target for antimetastatic drugs. Our study shows that cytochalasin H has the potential to inhibit cell migration as a secondary metabolite by blocking this protein. Molecular docking is a widely used method in drug discovery and cancer research to investigate the stable interaction between small molecules and proteins (20). This approach holds a valuable preclinical tool for the development of more effective and potent anticancer therapies (21).
Methods
The two-dimensional structure of cytochalasin H as a ligand was designed and optimized using the HyperChem 7.0 software (22) and the Polak-Ribiere algorithm to achieve the most stable structure. The ligand molecule in HIN format was converted to the Protein Data Bank (PDB) format using Open Babel 2.4.1 tool (22). This format is compatible with the PDB. The crystal structure of the human target protein fascin with a resolution of 2Å was obtained from the Brookhaven PDB (Code: 3p53). Discovery Studio software was used to remove water molecules and default ligands from the fascin protein structure. This step prepares the protein for further analysis. AutoDock Tools 1.5.6, part of the molecular graphics laboratory (MGL) software package, was used to convert the pre-processed fascin protein structure from PDB to PDB, Partial Charge (Q), and Atom Type (T) (PDBQT) format. PDBQT is the format required for AutoDock Vina, the docking software used in the study (23).
We performed rigid docking to predict atomic-level interaction between cytochalasin H and fascin protein by AutoDock Vina 1.1.2. (http://vina.scripps.edu). The results obtained from docking were analyzed using various software tools, such as LigPlot+ v.1.4.5, Discovery Studio 4.5, and PyMOL v.1.9. These tools provide visualization and analysis of the ligand-protein interactions, allowing for a more detailed understanding of the binding mode and potential binding sites.
Results
Cytochalasins have been found to impact various cellular functions, drug delivery, and chemotherapy. They can also induce a significant clinical response in cell systems as antitumor drugs (8). The molecular binding position between the ligand and protein was determined in the present study using a grid box. This function plays a crucial role in the selection and efficiency of molecular docking by restricting the search area in AutoDock. The coordinates of the grid box for the ligand binding region (actin-binding site) were set as 27.083 for X, -52.227 for Y, and 33.496 for Z. The size of the grid box was maintained at 42 * 34 * 40, with a grid spacing of 0.375 Å. The results obtained from AutoDock Vina 1.1.2 indicated that the affinity value for the interactions between fascin protein and cytochalasin H was determined to be -9.5 kcal/mol (Figure 1).

Cytochalasin H was docked to residues located in the active site of fascin protein. Analysis conducted using LigPlot+ v.1.4.5 software revealed the two-dimensional protein-ligand interactions, as shown in Figure 2. Amino acids present in the active site formed interactions with the ligand. The hydrophobic contacts involved Asp337, Val435, Thr434, Thr442, Gly436, Thr401, Thr403, Trp340, Cys456, Val485, Lys303, Ser444, and Lys304, which played a role in binding cytochalasin H to the fascin protein. Furthermore, three hydrogen bonds were observed with Arg348, Thr302, and Ile338. Among them, the hydrogen bond with Ile338 had a length of 2.93 Å and was related to a hydrophobic residue. Two hydrogen bonds, with lengths of 3.03 Å and 3.07 Å, were formed with the polar aliphatic residues Arg348 and Thr302, respectively, as depicted in Figure 2. The primary bonds responsible for stabilizing the ligand-enzyme complex were hydrogen bonds and hydrophobic interactions (24).


In this study, Discovery Studio 4.5 and PyMOL v.1.9 software was used for the three-dimensional analysis of molecular docking results. Figure 3a shows the ligand and protein interaction in the active site. Cytochalasin H is at its best energy level with the fascin protein. High-quality images were obtained from the ligand binding site to the protein. Figures 3a and 3b show an active site in the protein. This site is located at the interface between chain A and chain B.
Therefore, based on these results, cytochalasin H can be introduced as an effective antitumor agent in gastric cancer. However, further studies are needed to explore the exact mechanism.
Discussion
Given that gastric cancer is a multifactorial disease associated with high mortality rates, numerous studies have employed molecular docking to predict suitable drugs. In a study by Imani-Saber et al. in 2015, imatinib was proposed as a suitable ligand to inhibitcasein kinase 2 (CK2) and preserve promyelocytic leukemia (PML) as a tumor suppressor gene, focusing on gastric cancers caused by Helicobacter pylori (24). Also, much attention has been paid to fascin as a crucial actin-bundling protein (F-actin) pivotal in creating cell surface protrusions that facilitate cell migration (20,11). In normal adult epithelial tissues, fascin is typically absent or down-regulated. However, it is notably up-regulated in various carcinomas, demonstrating a positive correlation with invasion, metastasis, and unfavorable prognosis (11,25). Conversely, inhibiting fascin through an inhibitor can prevent the invasion of cancer cells. Multiple studies have investigated the effects of cytochalasins on different types of cancer or infectious diseases (26). The effects of cytochalasin H on apoptosis and migration were investigated in the human lung adenocarcinoma cell line A549. The treatment of A549 cells with cytochalasin H resulted in cell cycle arrest at the G2/M phase and showed strong anti-migratory activities (27). In our study, the main objective was to investigate and understand the underlying interactions through which cytochalasin H inhibits the migration ability of cancer cells, ultimately impeding the progression and metastasis of the disease. In line with this objective, we employed molecular docking techniques to investigate the potential of cytochalasin H as an inhibitor of fascin protein. These findings suggest that cytochalasin H may hold promise as a suitable inhibitor for targeting fascin. This study provides valuable insights into developing therapeutic strategies for gastric cancer treatment by elucidating the molecular interactions and binding energies. However, the precise determination of the inhibitor's concentration, dosage, and efficacy in inhibiting cancer cells requires additional in vitro and in vivo investigations.
Conclusion
In conclusion, targeting fascin represents a promising approach for controlling and treating gastric cancer. Inhibition of this protein probably can impact metastasis processes and cytoskeleton polymerization significantly. The docking results demonstrate that cytochalasin H has the potential to serve as a candidate drug for inhibiting and facilitating gastric cancer therapy. So, further investigations, including in vitro and in vivo studies, are warranted to validate these findings and explore the precise mechanism of action. The development of novel drug design strategies targeting fascin could pave the way for more effective treatments for gastric cancer, another cancer, or even the metastasis of other cancers by influencing the cellular skeleton.
Acknowledgement
Not applicable.
Funding sources
This study did not receive any funds.
Ethical statement
Not applicable. This article was conducted using software and data analysis.
Conflicts of interest
The authors declared no conflict of interest.
Author contributions
We confirm that all authors were involved in writing the article. S.H.: A master’s graduate, contribution in the manuscript conceptualization, writing, and introduction, and draft preparation; S.A.: A Ph.D. student, contribution in the manuscript methodology, software, writing, and results.
Type of Article: Original article |
Subject:
Molecular Sciences
Received: 2023/07/30 | Accepted: 2023/12/24 | Published: 2023/12/30
Received: 2023/07/30 | Accepted: 2023/12/24 | Published: 2023/12/30
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. 2019;14(1):26-38. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Fritz A, Editor. International Classification of Diseases for Oncology (ICD-O). 3rd ed. 1st Rev. WHO;2000:240 p. [View at Publisher] [Google Scholar]
4. Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56(1):1-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679-95. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Buday L, Downward J. Roles of cortactin in tumor pathogenesis. Biochim Biophys Acta. 2007;1775(2): 263-73. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda Sh, et al. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(7):2597-605. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Smith SC, Theodorescu D. Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer. 2009;9(4):253-64. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275-92. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Chen L, Yang Sh, Jakoncic J, Zhang J, Huang X. Migrastatin analogues target fascin to block tumour metastasis. Nature. 2010;464(7291):1062-66. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224(3):289-300. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67(3-4):262-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Van Goietsenoven G, Mathieu V, Andolfi A, Cimmino A, Lefranc F, Kiss R, et al. In vitro growth inhibitory effects of cytochalasins and derivatives in cancer cells. Planta Med. 2011;77(7):711- 17. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105(4):1473-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Haidle AM, Myers AG. An enantioselective, modular, and general route to the cytochalasins: synthesis of L-696,474 and cytochalasin B. Proc Natl Acad Sci U S A. 2004;101(33):12048-53. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Deshmukh PG, Kanitkar UK, Pendse GS. A new fungal isolate from Paspalum scrobiculatum, Linn, with new biologically active metabolites. Acta Microbiol Acad Sci Hung. 1975;22(3):253-62. [View at Publisher] [Google Scholar]
17. Chapla VM, Zeraik ML, Ximenes V, Zanardi LM, Lopes M, Cavalheiro A, et al. Bioactive secondary metabolites from Phomopsis sp., an endophytic fungus from Senna spectabilis. Molecules. 2014;19(5):6597-608. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Yi JM, Kim J, Park JS, Lee J, Lee YJ, Hong JT, et al. In vivo anti-tumor effects of the ethanol extract of gleditsia sinensis thorns and its active constituent, cytochalasin h. Biol Pharm Bull. 2015;38(6):909-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Jayo A, Parsons M. Fascin: A key regulator of cytoskeletal dynamics. Int J Biochem. Cell Biol. 2010;42(10):1614-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Mohd AR, Swaleha Z, Asim A. Ligand docking and binding site analysis with pymol and autodock/vina. International Journal of Basic and Applied Sciences. 2015;4(2):168-77. [View at Publisher] [DOI] [Google Scholar]
21. Phillips MA, Stewart MA, Woodling DL, Xie ZR. Has Molecular Docking Ever Brought us a Medicine? BoD – Books on Demand;2018. [View at Publisher] [DOI] [Google Scholar]
22. Zolfaghari N. Molecular docking analysis of nitisinone with homogentisate 1,2 dioxygenase. Bioinformation. 2017;13(5):136-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Xiong SL, Yue LM, Lim GT, Yang JM, Lee J, Park YD, et al. Inhibitory effect of raspberry ketone on α-glucosidase: Docking simulation integrating inhibition kinetics. Int J Biol Macromol. 2018;113:212-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Imani-Saber Z, Ghafouri-Fard S. In Silico Interaction and Docking Studies Indicate a New Mechanism for PML Dysfunction in Gastric Cancer and Suggest Imatinib as a Drug to Restore Function. Asian Pac J Cancer Prev. 2015;16(12):5005-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 2008;56(2):193-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Feitosa A, Ferreira F, Brigido H, Bastos M, Carvalho J, Carneiro A, et al. Study on Experimental Leishmanicidal Activity and in silico of Cytochalasin B. J Braz Chem Soc. 2019;30(3):592-6. [View at Publisher] [Google Scholar]
27. Ma Y, Wu X, Xiu Z, Liu X, Huang B, Hu L, et al. Cytochalasin H isolated from mangrove derived endophytic fungus induces apoptosis and inhibits migration in lung cancer cells. Oncol Rep. 2018;39(6):2899-905. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






