Volume 11, Issue 1 (7-2023)
Jorjani Biomed J 2023, 11(1): 20-24 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shafabakhsh S R, kazemzadeh Y, Shirvani H, Mirzaiyan Shanjani S, Sedaghati S. Investigating the impact of aerobic training on myokine gene expression in the skeletal muscle of wistar rats. Jorjani Biomed J 2023; 11 (1) :20-24
URL: http://goums.ac.ir/jorjanijournal/article-1-934-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-934-en.html
Seyed Rafie Shafabakhsh1 

 , Yaser Kazemzadeh2
, Yaser Kazemzadeh2 

 , Hossein Shirvani3
, Hossein Shirvani3 

 , Sanaz Mirzaiyan Shanjani1
, Sanaz Mirzaiyan Shanjani1 

 , Saeed Sedaghati4
, Saeed Sedaghati4 




 , Yaser Kazemzadeh2
, Yaser Kazemzadeh2 

 , Hossein Shirvani3
, Hossein Shirvani3 

 , Sanaz Mirzaiyan Shanjani1
, Sanaz Mirzaiyan Shanjani1 

 , Saeed Sedaghati4
, Saeed Sedaghati4 


1- Department of Sports Physiology, Faculty of Physical Education, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran
2- Department of Sports Physiology, Faculty of Physical Education, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran ,Yaser.kazemzadeh@yahoo.com
3- Exercise Physiology Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
4- Department of Sports Management, Faculty of Physical Education, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran
2- Department of Sports Physiology, Faculty of Physical Education, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran ,
3- Exercise Physiology Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
4- Department of Sports Management, Faculty of Physical Education, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran
Keywords: Moderate intensity aerobic exercise, Apelin, Decorin, Musclin protein, Energy homeostasis associated protein
Full-Text [PDF 559 kb]
(2152 Downloads)
| Abstract (HTML) (6773 Views)
Full-Text: (1313 Views)
Introduction
Skeletal muscle is a fully vascularized tissue that can secrete proteins/peptides (1) from muscle cells under the influence of training stimuli and in response to muscle contractions or sports activity (2, 3). These productions and secretions of skeletal muscles are called myokines (2) to emphasize their muscular origin (1). Myokines are peptides secreted from skeletal muscle cells and affect other cells, tissues, and organs in a paracrine and endocrine manner. Besides, they exert their autocrine effects on the same muscle or other muscles (3, 4).
Muscles, specifically skeletal and cardiac, are critical mediators of systemic energy homeostasis (6, 5). As an energy-consuming tissue, skeletal muscle regulates the energy homeostasis of the whole body through the energy requirements for active muscles (7). Beyond energy substrate consumption, the muscle can also secrete humoral factors to regulate systemic energy homeostasis through intertissued communication. As an endocrine organ, the muscle can regulate and modulate metabolic changes in other tissues, such as the liver, adipose, and pancreas, through gene expression and secretion of numerous proteins (myokines), as well as metabolic substrates and mediators. Myokines can direct energy homeostasis independently or cooperatively by regulating multiple aspects of glucose metabolism, lipid metabolism, and insulin sensitivity in other tissues (5, 6, 8, 9, 10). Therefore, muscle serves as a key endocrine and metabolic mediator of systemic lipid metabolism required to control energy homeostasis, and understanding how muscle communicates with adipose storage tissues to coordinate energy expenditure remains a critical question.
Decorin is one of the components of connective tissue attached to the narrow strands of collagen type 1. It participates in constructing the extracellular matrix during muscle contraction following exercise, and its expression, secretion, and level increase (11). Undoubtedly, the signaling pathways involved in decorin expression within skeletal muscles work so that engaging in sports activities triggers the secretion of decorin. This substance then binds to its receptor, known as ALK4, 5 ACTR II, as well as Smad2/3. Subsequently, it undergoes phosphorylation and activation. By binding to Smad4, decorin enters the cell nucleus and activates PGC-1α, increasing the mRNA associated with this myokine (12). The results show that decorin plays an essential role in cell growth by modulating the activity of growth factors and modifying the growth activity of various cells through interaction with TGF-β (13). Because of its anti-fibrotic and anti-inflammatory effects, decorin may be a potential therapeutic target for diseases related to muscle wasting, as well as an emerging therapeutic candidate for patients with solid malignancies (14).
Musclin is another exercise-induced myokine expressed in skeletal muscles more than ten times compared to other tissues such as bone, brown fat, testis, and spleen, and it plays a crucial role in energy homeostasis (12). Investigations have revealed that muscle contractions (such as those during physical activity) activate the signaling pathways of musclin gene expression in muscle cells, leading to increased levels of musclin. When musclin binds to its receptor, it stimulates the natriuretic peptide (NPR) and activates cGAP. This, in turn, triggers PGC-1α, resulting in a rise in musclin mRNA levels. By following the autocrine, paracrine, and endocrine patterns, musclin can significantly regulate, multiply, and differentiate muscle cells, control inflammation and insulin resistance, and suppress tumor growth (15, 12). Some researchers have considered sports activity as a factor in reducing the circulating levels of musclin; others believe that the musclin gene is expressed by physical activity, even daily activities. It also increases after intense activities, emphasizing its role in the process of mitochondrial biogenesis and, as a result, energy production and balance in the body (16, 15).
Apelin is an exercise-regulated myokine with autocrine/paracrine activity in skeletal muscle (17). Apelin levels in muscle and plasma are regulated by exercise and improve metabolism in obese or T2D mice and humans (12). Endurance training has been shown to increase apelin expression in the skeletal muscle of obese men. Furthermore, apelin is expressed by fat cells and is associated with energy homeostasis in metabolic diseases (18, 17).
The results of the studies show that exercise can increase the secretion of myokines such as musclin, apelin, and decorin in the blood serum and their gene expression in the muscle. The results of the studies on decorin, musclin, apelin, and energy homeostasis are limited and contradictory. Therefore, expanding the understanding about which activities with different intensities can play a role in the secretion, primarily expressing specific myokines in the muscle, can effectively regulate the body's metabolism, energy production, and homeostasis. Besides, it is critical in preventing, or treating many diseases, even in the prescription of more appropriate exercise in the repair of some specific conditions or drugs based on myokines can be valuable and practical, it seems necessary. According to the above material, the importance and necessity of conducting new research in this direction has doubled. Accordingly, this study aimed to investigate the effect of 4-week moderate-intensity aerobic training on the expression of apelin, decorin, and musclin genes in the slow twitch fibers of skeletal muscle of male Wistar rats.
Methods
This study was experimental research conducted in a laboratory and controlled manner. Given that it was impossible to access human subjects due to space, ethical, and time constraints, animal subjects (male Wistar rats) were used. After obtaining the necessary permits, rats were purchased from the Pasteur Laboratory Animal Breeding and Reproduction Center (Tehran, Iran) and transferred to the animal laboratory of the Sports Physiology Center of Baqiyatallah University of Medical Sciences. They were then kept in separate cages according to the instructions of the Iranian Society for the Protection of Laboratory Animals. Rats were kept in individual cages in an environment with an average temperature of 22±1.4 degrees Celsius, 55±4 percent humidity, and a 12:12 light-dark cycle according to the instructions of the Iranian Society for the Protection of Laboratory Animals. Likewise, rats had free access to standard laboratory food and water throughout the research. All ethical principles were observed under the principles of working with laboratory animals and human studies approved by Baqiyatallah University of Medical Sciences.
Sampling and intervention
The statistical sample consisted of 16 Wistar male rats with an approximate 8-week age and a weight range of 200-220 grams, randomly divided into two groups: An aerobic exercise group (n=8) and a control group (n=8). The sample size was determined using G POWER software based on the statistical analysis method of variance and alpha error level of 0.05 and power of 0.85.
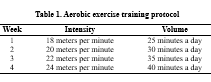
The exercise training protocol included 4-week moderate-intensity aerobic exercises with a frequency of 3 sessions per week. In the first week, the experimental group ran on the treadmill at a speed of 18 meters per minute for 25 minutes in each session. Then, every week, the volume of training (training time in each session) and training intensity (running speed) increased progressively until the fourth week (19). The details of the training protocol are provided in Table 1.
A one-week course was conducted to familiarize with the laboratory environment and exercise training protocol. The familiarization training protocol included a 3- consecutive training day for 10-15 minutes of running at a speed of 5-7 meters per minute on a treadmill. Maximum power was used to control the training intensity. For this purpose, the standard incremental test of Biddeford et al. (1979) (20), standardized by Leandro et al. (2007) (21) for rats and mice, was used. The incremental test consisted of a ten-step non-inclined treadmill running test, run after ten minutes of warm-up at low speed. The starting speed was 5 m/min; every three minutes, the speed was increased by 5 m/min until the rats could no longer run. The final speed was obtained as the maximum speed when reaching the maximum power, and 50 and 70% of it were determined as the minimum and maximum training intensities. Immediately after a day of rest, the 4-week training program was performed by the experimental group. During this period, the control group did not engage in sports activities.
Measuring gene expression
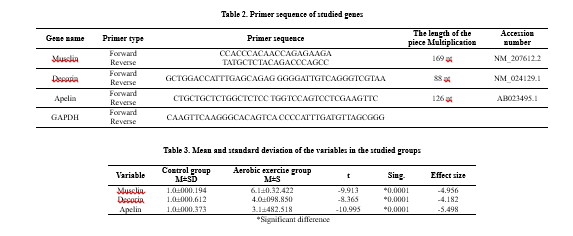
After completing four weeks of intervention, after the last session of training and maintenance, the animals were taken to the laboratory and sacrificed. The plantar soleus muscle sample was taken to examine the expression of genes. In each group, tissues were examined with the Real-Time PCR technique. First, the primer design was done, and total RNA was extracted from the tissues and converted into cDNA. The cDNA was then amplified by PCR and analyzed for the genes' expression. For molecular investigations at the gene expression level, first, RNA extraction from tissues in all investigated groups was performed according to the manufacturer's protocol (Qiagen, Germany). The Comparative ΔΔCt method was used to quantify the expression of apelin mRNA, muscle mRNA, and decorin mRNA. In this ΔΔCt method, the following relationship was used: ΔΔCt = ΔCt sample - ΔCt control
In this regard, ΔCt sample is the difference between the threshold cycles of the desired gene and the housekeeper in the tested sample (patient), and ΔCt control is the difference between the threshold cycles of the desired gene and the housekeeper in the control sample (healthy). The resulting number is placed in the relation ΔΔCt-2, which shows the difference in expression between the control sample and the test case (Table 2).
Statistical Methods
At the level of descriptive statistics, indicators such as mean, standard deviation, frequency distribution tables, and then the Kolmogorov-Smirnov test were used to check the normality of data distribution. An independent t-test was used for data analysis in the inferential statistics section. An alpha level of less than 0.05 was considered a significant level. The collected data were statistically analyzed by SPSS version 27 software.
Results
The results of the expression of apelin, decorin, and muslin genes are reported as mean, standard deviation, and comparison of gene expression between the control and aerobic training groups (Table 3).
The results obtained from the independent t-test showed that the gene expression of all three variables, muslin (P=0.0001), decorin (P=0.0001), and apelin (P=0.0001) in the aerobic training group was significantly higher than in the control group.

Discussion
The present study investigated the effect of moderate-intensity aerobic exercise on the expression of apelin, decorin, and musclin genes in the skeletal muscle of male Wistar rats. To further understand the potential role of myokines in fundamental aspects of variation in the adaptive response to exercise training (exercise capacity), this study used an experimental design in male Wistar rats.
The research findings indicate that the expression values of apelin, decorin, and muslin genes in the training group were significantly higher than in the control group.
The muscle acts as a fundamental endocrine and metabolic mediator of systemic lipid metabolism required to control energy homeostasis by secreting various factors called myokines (6, 22), which can be altered by exercise (23). Musclin is one of the exercise-induced myokines expressed several times more in skeletal muscles than in other tissues and plays an essential role in energy homeostasis (24). Recent studies indicated that mice without musclin have a lower physical capacity (25), and decreased expression of musclin was also observed in obese mice after exercise (26). Yu et al. (2016) revealed that 8-week aerobic and resistance training decreased basal levels of musclin gene expression with increased GLUT-4 expression in the skeletal muscle of obese mice fed a high-fat diet, inconsistent with the results of the present study (26). The type of exercise (endurance or resistance), duration, and diet are the reasons for the difference in the results of different studies (26). In humans, the musclin response to exercise is not known. Indicatively, musclin responds to daily and intense physical activity, indicating the role of musclin in mitochondrial biogenesis and energy balance (25). Most studies have focused on the role of musclin on insulin resistance. Therefore, its results are in line with the results of this research. So, an increase in musclin mRNA expression was observed in the training group, and this increase in response to aerobic exercise was caused by an increase in musclin gene expression in the soleus muscle tissue, occurring during the activation of PGC1-α in muscle cells.
Aplin is a myokine involved in the pathophysiology of obesity, IR, type 2 diabetes, and cardiovascular function (27). Currently, controversy has been observed regarding the response of apelin concentration after exercise interventions (18, 28). While a study involving patients with diabetes reported an increase in circulating apelin levels after continuous aerobic training (26), another investigation showed no change after a similar exercise program (18).
The apelin/apelin receptor system plays an essential role in glucose metabolism and insulin sensitivity. Low-dose intravenous apelin can lower blood glucose and improve glucose tolerance in healthy rats. When hepatic glucose production is entirely inhibited during the hyperinsulinemic-euglycemic clamp, apelin can decrease blood glucose by increasing glucose uptake from skeletal muscle and adipose tissue, thereby increasing total glucose consumption (29, 30, 31).
Skeletal muscle plays an essential role in maintaining normal glucose homeostasis and regulating whole-body carbohydrate metabolism. Apelin can increase glucose transport in isolated soleus muscle (29, 30) and synergize with insulin. Studies have shown that the effect of apelin on reducing glucose levels depends on the activation of AMPK and eNOS (30, 32). However, apelin has been reported to increase glucose uptake mainly through the AMPK pathway rather than the eNOS pathway in cultured C2C12 muscle cells (33). In addition, apelin can increase the phosphorylation of Akt in isolated C2C12 myotubes (30, 34). Notably, plasma apelin concentrations increased in obese and insulin-resistant rats fed a high-fat diet, while intravenous apelin injection during the hyperinsulinemic-euglycemic clamp remained effective in improving insulin sensitivity (29). Therefore, exogenous administration of apelin can significantly improve glucose tolerance and insulin sensitivity, mainly dependent on improving skeletal muscle metabolic functions, in addition to increased skeletal muscle glucose uptake. It was found that apelin knockout mice showed increased insulin resistance during high-fat diet feeding, further confirming the role of apelin in regulating glucose homeostasis (33). In human adipose tissue explants, apelin can stimulate glucose transport in an AMPK-dependent manner, while 3 T3-L1 adipocytes can achieve the same result by activating the PI3K/Akt signaling pathway. In addition, apelin increased insulin-stimulated glucose transport in insulin-resistant T3-L1 cells (35).
In addition to skeletal muscle and adipose tissue, apelin increased glucose uptake and Glut4 membrane translocation in the myocardium of C57BL/6 J mice in vivo. According to the results of laboratory experiments, apelin also increased glucose transport in the H9C2 embryonic cardiomyocyte cell line (30, 34).
Glucose can rapidly stimulate apelin secretion in mouse intestinal epithelial cells. Aplin increases the net flow of glucose across the mucosal barrier of the gastrointestinal tract. Moreover, phosphorylating AMPK increases glucose transport from the intestinal lumen into the blood and regulates the ratio of sodium-glucose transporter 1 (SGLT-1) to glucose transporter 2 (GLUT2). Conversely, administering an apelin blocker can reduce hyperglycemia after oral glucose administration. Glucose stimulates apelin secretion in the intestinal epithelial cells, and apelin increases glucose transport from the intestinal tract to the blood. The interaction between glucose and apelin increases portal vein glucose levels, stimulating rapid insulin secretion and improving insulin sensitivity (36, 37). Therefore, apelin can also maintain glucose homeostasis by increasing intestinal epithelial glucose uptake, thereby increasing portal blood glucose and insulin secretion. A study showed an increase in muscle apelin gene expression in response to exercise and stated that this increase is positively related to improving insulin sensitivity (6). The results of another study have shown that endurance training can play a role in increasing the expression of the apelin gene in the skeletal muscles of obese men. Fat cells also express it and can affect energy homeostasis in metabolic disease (6, 27). Therefore, in line with previous studies, the results of the present study also showed that the increase in apelin mRNA expression in response to aerobic exercise is caused by the increase in the level of apelin gene expression in the soleus muscle tissue, after the activation of PGC1-a in muscle cells.
Studies have yet to be conducted on the effect of sports activity on decorin levels, and different results have been obtained. Some have confirmed the positive effect of sports activity (38), and some have not observed any change in the amount of decorin (39). Correspondingly, in most studies, the changes of decorin in skeletal muscle tendons have been investigated, and the information related to the regulation of decorin in the growth of skeletal muscles in response to exercise is limited (40) and was investigated for the first time by Tosta Kanzleiter (2014) (11). On the other hand, few studies have addressed the effect of different intensities of sports activity on decorin levels in skeletal muscle. Only Zhu et al. (2018) investigated the effect of eight weeks of exercise with different intensities on the expression of decorin in the Achilles tendon of Wistar rats. The results indicated an increase in the expression of decorin in the moderate-intensity exercise group compared to the control group and a decrease in the expression of decorin in the high-intensity exercise group compared to the control and moderate-intensity groups. This may explain why moderate-intensity exercise increases tendon strength and tensile strength, and intense exercise decreases decorin levels and decreases tendon strength and stiffness (41). Seemingly, the difference between the study's results by Zhu et al. (2018) and the present study can be attributed to the investigated tissue (tendon and muscle) and different intensities of sports activity in these studies. Furthermore, in line with a limited number of studies, the results of the present study also showed that after 4-week aerobic training, decorin mRNA expression increases through phosphorylation and activation of Smad2/3 and activation of PGC1-a. Therefore, regarding the expression of the physiological mechanism of the effect of exercise on decorin expression, intervening factors such as the intensity and duration of exercise, muscle damage, the type of subjects, the method of examining gene expression, and the type of target tissue (tendon and muscle) can be effective. The effect mechanisms of sports training on decorin are complex and require more studies.
Demonstratively, the difference in the results of the studies can be attributed to the type of skeletal muscles (slow-twitch and fast-twitch) involved and the type of sports activity —no research related to the effect of different intensities of sports activity that simultaneously measured decorin levels. The study of Potter et al. (2017) examined the effect of exercise on TGF-β and decorin levels. Besides, the results indicated increased TGF-β levels in the training group and no change in decorin gene expression in the Achilles tendon of male Wistar rats (42). Sotoudeh et al. (2017) investigated the decorin gene expression in the soleus muscle as a result of eight weeks of intermittent aerobic exercise in an animal model of breast cancer. In addition, the study's results indicated an increase in decorin levels in the soleus muscle (2). This research's results are consistent with the results of the current research.
Skeletal muscle is an endocrine organ that not only contains essentialmetabolic molecules whose expression changes metabolism but also communicates with other tissues through the secretion of hormones known as myokines.
The co-activating factor of peroxisome proliferator-activated receptor-gamma (PGC1-a) is one of the crucial metabolic molecules expressed in muscle. It plays an essential role in maintaining glucose, lipid, and energy homeostasis, and its expression increases with exercise in skeletal muscle (43). Myokines are probably a bridge between the interaction between skeletal muscle and other tissues involved in homeostasis and energy metabolism, including fat tissue (8).
Therefore, regarding the expression of the physiological mechanism of the effect of exercise training on the expression of muslin, aplin, and decorin genes, seemingly, intervening factors such as the intensity and duration of exercise, muscle damage, the type of subjects, the method of examining gene expression, and the type of tissue, (tendon and muscle) can be the reason for the difference in the studies.
Conclusion
The present research is conducted in muslin, apelin, and decorin gene expression using rat samples with aerobic exercise, which is vital. In general, the results of this study showed that four weeks of moderate-intensity aerobic exercise significantly increased the gene expression of the studied variables. According to the present study's findings, performing aerobic exercise training by increasing the expression of musclin, apelin, and decorin plays an essential role in energy homeostasis, showing the importance of this type of exercise in health.
Acknowledgments
This article is taken from the thesis of a doctoral degree in exercise physiology student with the code of ethics IR.IAU.PIAU.REC.1401.009 in the Faculty of Physical Education, Islamic Azad University, Islamshahr Branch. The researchers express their utmost gratitude to the officials of the Islamic Azad University, Islamshahr branch, who helped us conduct this research with their participation, and to all the people who collaborated in this study.
Skeletal muscle is a fully vascularized tissue that can secrete proteins/peptides (1) from muscle cells under the influence of training stimuli and in response to muscle contractions or sports activity (2, 3). These productions and secretions of skeletal muscles are called myokines (2) to emphasize their muscular origin (1). Myokines are peptides secreted from skeletal muscle cells and affect other cells, tissues, and organs in a paracrine and endocrine manner. Besides, they exert their autocrine effects on the same muscle or other muscles (3, 4).
Muscles, specifically skeletal and cardiac, are critical mediators of systemic energy homeostasis (6, 5). As an energy-consuming tissue, skeletal muscle regulates the energy homeostasis of the whole body through the energy requirements for active muscles (7). Beyond energy substrate consumption, the muscle can also secrete humoral factors to regulate systemic energy homeostasis through intertissued communication. As an endocrine organ, the muscle can regulate and modulate metabolic changes in other tissues, such as the liver, adipose, and pancreas, through gene expression and secretion of numerous proteins (myokines), as well as metabolic substrates and mediators. Myokines can direct energy homeostasis independently or cooperatively by regulating multiple aspects of glucose metabolism, lipid metabolism, and insulin sensitivity in other tissues (5, 6, 8, 9, 10). Therefore, muscle serves as a key endocrine and metabolic mediator of systemic lipid metabolism required to control energy homeostasis, and understanding how muscle communicates with adipose storage tissues to coordinate energy expenditure remains a critical question.
Decorin is one of the components of connective tissue attached to the narrow strands of collagen type 1. It participates in constructing the extracellular matrix during muscle contraction following exercise, and its expression, secretion, and level increase (11). Undoubtedly, the signaling pathways involved in decorin expression within skeletal muscles work so that engaging in sports activities triggers the secretion of decorin. This substance then binds to its receptor, known as ALK4, 5 ACTR II, as well as Smad2/3. Subsequently, it undergoes phosphorylation and activation. By binding to Smad4, decorin enters the cell nucleus and activates PGC-1α, increasing the mRNA associated with this myokine (12). The results show that decorin plays an essential role in cell growth by modulating the activity of growth factors and modifying the growth activity of various cells through interaction with TGF-β (13). Because of its anti-fibrotic and anti-inflammatory effects, decorin may be a potential therapeutic target for diseases related to muscle wasting, as well as an emerging therapeutic candidate for patients with solid malignancies (14).
Musclin is another exercise-induced myokine expressed in skeletal muscles more than ten times compared to other tissues such as bone, brown fat, testis, and spleen, and it plays a crucial role in energy homeostasis (12). Investigations have revealed that muscle contractions (such as those during physical activity) activate the signaling pathways of musclin gene expression in muscle cells, leading to increased levels of musclin. When musclin binds to its receptor, it stimulates the natriuretic peptide (NPR) and activates cGAP. This, in turn, triggers PGC-1α, resulting in a rise in musclin mRNA levels. By following the autocrine, paracrine, and endocrine patterns, musclin can significantly regulate, multiply, and differentiate muscle cells, control inflammation and insulin resistance, and suppress tumor growth (15, 12). Some researchers have considered sports activity as a factor in reducing the circulating levels of musclin; others believe that the musclin gene is expressed by physical activity, even daily activities. It also increases after intense activities, emphasizing its role in the process of mitochondrial biogenesis and, as a result, energy production and balance in the body (16, 15).
Apelin is an exercise-regulated myokine with autocrine/paracrine activity in skeletal muscle (17). Apelin levels in muscle and plasma are regulated by exercise and improve metabolism in obese or T2D mice and humans (12). Endurance training has been shown to increase apelin expression in the skeletal muscle of obese men. Furthermore, apelin is expressed by fat cells and is associated with energy homeostasis in metabolic diseases (18, 17).
The results of the studies show that exercise can increase the secretion of myokines such as musclin, apelin, and decorin in the blood serum and their gene expression in the muscle. The results of the studies on decorin, musclin, apelin, and energy homeostasis are limited and contradictory. Therefore, expanding the understanding about which activities with different intensities can play a role in the secretion, primarily expressing specific myokines in the muscle, can effectively regulate the body's metabolism, energy production, and homeostasis. Besides, it is critical in preventing, or treating many diseases, even in the prescription of more appropriate exercise in the repair of some specific conditions or drugs based on myokines can be valuable and practical, it seems necessary. According to the above material, the importance and necessity of conducting new research in this direction has doubled. Accordingly, this study aimed to investigate the effect of 4-week moderate-intensity aerobic training on the expression of apelin, decorin, and musclin genes in the slow twitch fibers of skeletal muscle of male Wistar rats.
Methods
This study was experimental research conducted in a laboratory and controlled manner. Given that it was impossible to access human subjects due to space, ethical, and time constraints, animal subjects (male Wistar rats) were used. After obtaining the necessary permits, rats were purchased from the Pasteur Laboratory Animal Breeding and Reproduction Center (Tehran, Iran) and transferred to the animal laboratory of the Sports Physiology Center of Baqiyatallah University of Medical Sciences. They were then kept in separate cages according to the instructions of the Iranian Society for the Protection of Laboratory Animals. Rats were kept in individual cages in an environment with an average temperature of 22±1.4 degrees Celsius, 55±4 percent humidity, and a 12:12 light-dark cycle according to the instructions of the Iranian Society for the Protection of Laboratory Animals. Likewise, rats had free access to standard laboratory food and water throughout the research. All ethical principles were observed under the principles of working with laboratory animals and human studies approved by Baqiyatallah University of Medical Sciences.
Sampling and intervention
The statistical sample consisted of 16 Wistar male rats with an approximate 8-week age and a weight range of 200-220 grams, randomly divided into two groups: An aerobic exercise group (n=8) and a control group (n=8). The sample size was determined using G POWER software based on the statistical analysis method of variance and alpha error level of 0.05 and power of 0.85.
The exercise training protocol included 4-week moderate-intensity aerobic exercises with a frequency of 3 sessions per week. In the first week, the experimental group ran on the treadmill at a speed of 18 meters per minute for 25 minutes in each session. Then, every week, the volume of training (training time in each session) and training intensity (running speed) increased progressively until the fourth week (19). The details of the training protocol are provided in Table 1.

A one-week course was conducted to familiarize with the laboratory environment and exercise training protocol. The familiarization training protocol included a 3- consecutive training day for 10-15 minutes of running at a speed of 5-7 meters per minute on a treadmill. Maximum power was used to control the training intensity. For this purpose, the standard incremental test of Biddeford et al. (1979) (20), standardized by Leandro et al. (2007) (21) for rats and mice, was used. The incremental test consisted of a ten-step non-inclined treadmill running test, run after ten minutes of warm-up at low speed. The starting speed was 5 m/min; every three minutes, the speed was increased by 5 m/min until the rats could no longer run. The final speed was obtained as the maximum speed when reaching the maximum power, and 50 and 70% of it were determined as the minimum and maximum training intensities. Immediately after a day of rest, the 4-week training program was performed by the experimental group. During this period, the control group did not engage in sports activities.
Measuring gene expression
After completing four weeks of intervention, after the last session of training and maintenance, the animals were taken to the laboratory and sacrificed. The plantar soleus muscle sample was taken to examine the expression of genes. In each group, tissues were examined with the Real-Time PCR technique. First, the primer design was done, and total RNA was extracted from the tissues and converted into cDNA. The cDNA was then amplified by PCR and analyzed for the genes' expression. For molecular investigations at the gene expression level, first, RNA extraction from tissues in all investigated groups was performed according to the manufacturer's protocol (Qiagen, Germany). The Comparative ΔΔCt method was used to quantify the expression of apelin mRNA, muscle mRNA, and decorin mRNA. In this ΔΔCt method, the following relationship was used: ΔΔCt = ΔCt sample - ΔCt control
In this regard, ΔCt sample is the difference between the threshold cycles of the desired gene and the housekeeper in the tested sample (patient), and ΔCt control is the difference between the threshold cycles of the desired gene and the housekeeper in the control sample (healthy). The resulting number is placed in the relation ΔΔCt-2, which shows the difference in expression between the control sample and the test case (Table 2).
Statistical Methods
At the level of descriptive statistics, indicators such as mean, standard deviation, frequency distribution tables, and then the Kolmogorov-Smirnov test were used to check the normality of data distribution. An independent t-test was used for data analysis in the inferential statistics section. An alpha level of less than 0.05 was considered a significant level. The collected data were statistically analyzed by SPSS version 27 software.
Results
The results of the expression of apelin, decorin, and muslin genes are reported as mean, standard deviation, and comparison of gene expression between the control and aerobic training groups (Table 3).
The results obtained from the independent t-test showed that the gene expression of all three variables, muslin (P=0.0001), decorin (P=0.0001), and apelin (P=0.0001) in the aerobic training group was significantly higher than in the control group.


Discussion
The present study investigated the effect of moderate-intensity aerobic exercise on the expression of apelin, decorin, and musclin genes in the skeletal muscle of male Wistar rats. To further understand the potential role of myokines in fundamental aspects of variation in the adaptive response to exercise training (exercise capacity), this study used an experimental design in male Wistar rats.
The research findings indicate that the expression values of apelin, decorin, and muslin genes in the training group were significantly higher than in the control group.
The muscle acts as a fundamental endocrine and metabolic mediator of systemic lipid metabolism required to control energy homeostasis by secreting various factors called myokines (6, 22), which can be altered by exercise (23). Musclin is one of the exercise-induced myokines expressed several times more in skeletal muscles than in other tissues and plays an essential role in energy homeostasis (24). Recent studies indicated that mice without musclin have a lower physical capacity (25), and decreased expression of musclin was also observed in obese mice after exercise (26). Yu et al. (2016) revealed that 8-week aerobic and resistance training decreased basal levels of musclin gene expression with increased GLUT-4 expression in the skeletal muscle of obese mice fed a high-fat diet, inconsistent with the results of the present study (26). The type of exercise (endurance or resistance), duration, and diet are the reasons for the difference in the results of different studies (26). In humans, the musclin response to exercise is not known. Indicatively, musclin responds to daily and intense physical activity, indicating the role of musclin in mitochondrial biogenesis and energy balance (25). Most studies have focused on the role of musclin on insulin resistance. Therefore, its results are in line with the results of this research. So, an increase in musclin mRNA expression was observed in the training group, and this increase in response to aerobic exercise was caused by an increase in musclin gene expression in the soleus muscle tissue, occurring during the activation of PGC1-α in muscle cells.
Aplin is a myokine involved in the pathophysiology of obesity, IR, type 2 diabetes, and cardiovascular function (27). Currently, controversy has been observed regarding the response of apelin concentration after exercise interventions (18, 28). While a study involving patients with diabetes reported an increase in circulating apelin levels after continuous aerobic training (26), another investigation showed no change after a similar exercise program (18).
The apelin/apelin receptor system plays an essential role in glucose metabolism and insulin sensitivity. Low-dose intravenous apelin can lower blood glucose and improve glucose tolerance in healthy rats. When hepatic glucose production is entirely inhibited during the hyperinsulinemic-euglycemic clamp, apelin can decrease blood glucose by increasing glucose uptake from skeletal muscle and adipose tissue, thereby increasing total glucose consumption (29, 30, 31).
Skeletal muscle plays an essential role in maintaining normal glucose homeostasis and regulating whole-body carbohydrate metabolism. Apelin can increase glucose transport in isolated soleus muscle (29, 30) and synergize with insulin. Studies have shown that the effect of apelin on reducing glucose levels depends on the activation of AMPK and eNOS (30, 32). However, apelin has been reported to increase glucose uptake mainly through the AMPK pathway rather than the eNOS pathway in cultured C2C12 muscle cells (33). In addition, apelin can increase the phosphorylation of Akt in isolated C2C12 myotubes (30, 34). Notably, plasma apelin concentrations increased in obese and insulin-resistant rats fed a high-fat diet, while intravenous apelin injection during the hyperinsulinemic-euglycemic clamp remained effective in improving insulin sensitivity (29). Therefore, exogenous administration of apelin can significantly improve glucose tolerance and insulin sensitivity, mainly dependent on improving skeletal muscle metabolic functions, in addition to increased skeletal muscle glucose uptake. It was found that apelin knockout mice showed increased insulin resistance during high-fat diet feeding, further confirming the role of apelin in regulating glucose homeostasis (33). In human adipose tissue explants, apelin can stimulate glucose transport in an AMPK-dependent manner, while 3 T3-L1 adipocytes can achieve the same result by activating the PI3K/Akt signaling pathway. In addition, apelin increased insulin-stimulated glucose transport in insulin-resistant T3-L1 cells (35).
In addition to skeletal muscle and adipose tissue, apelin increased glucose uptake and Glut4 membrane translocation in the myocardium of C57BL/6 J mice in vivo. According to the results of laboratory experiments, apelin also increased glucose transport in the H9C2 embryonic cardiomyocyte cell line (30, 34).
Glucose can rapidly stimulate apelin secretion in mouse intestinal epithelial cells. Aplin increases the net flow of glucose across the mucosal barrier of the gastrointestinal tract. Moreover, phosphorylating AMPK increases glucose transport from the intestinal lumen into the blood and regulates the ratio of sodium-glucose transporter 1 (SGLT-1) to glucose transporter 2 (GLUT2). Conversely, administering an apelin blocker can reduce hyperglycemia after oral glucose administration. Glucose stimulates apelin secretion in the intestinal epithelial cells, and apelin increases glucose transport from the intestinal tract to the blood. The interaction between glucose and apelin increases portal vein glucose levels, stimulating rapid insulin secretion and improving insulin sensitivity (36, 37). Therefore, apelin can also maintain glucose homeostasis by increasing intestinal epithelial glucose uptake, thereby increasing portal blood glucose and insulin secretion. A study showed an increase in muscle apelin gene expression in response to exercise and stated that this increase is positively related to improving insulin sensitivity (6). The results of another study have shown that endurance training can play a role in increasing the expression of the apelin gene in the skeletal muscles of obese men. Fat cells also express it and can affect energy homeostasis in metabolic disease (6, 27). Therefore, in line with previous studies, the results of the present study also showed that the increase in apelin mRNA expression in response to aerobic exercise is caused by the increase in the level of apelin gene expression in the soleus muscle tissue, after the activation of PGC1-a in muscle cells.
Studies have yet to be conducted on the effect of sports activity on decorin levels, and different results have been obtained. Some have confirmed the positive effect of sports activity (38), and some have not observed any change in the amount of decorin (39). Correspondingly, in most studies, the changes of decorin in skeletal muscle tendons have been investigated, and the information related to the regulation of decorin in the growth of skeletal muscles in response to exercise is limited (40) and was investigated for the first time by Tosta Kanzleiter (2014) (11). On the other hand, few studies have addressed the effect of different intensities of sports activity on decorin levels in skeletal muscle. Only Zhu et al. (2018) investigated the effect of eight weeks of exercise with different intensities on the expression of decorin in the Achilles tendon of Wistar rats. The results indicated an increase in the expression of decorin in the moderate-intensity exercise group compared to the control group and a decrease in the expression of decorin in the high-intensity exercise group compared to the control and moderate-intensity groups. This may explain why moderate-intensity exercise increases tendon strength and tensile strength, and intense exercise decreases decorin levels and decreases tendon strength and stiffness (41). Seemingly, the difference between the study's results by Zhu et al. (2018) and the present study can be attributed to the investigated tissue (tendon and muscle) and different intensities of sports activity in these studies. Furthermore, in line with a limited number of studies, the results of the present study also showed that after 4-week aerobic training, decorin mRNA expression increases through phosphorylation and activation of Smad2/3 and activation of PGC1-a. Therefore, regarding the expression of the physiological mechanism of the effect of exercise on decorin expression, intervening factors such as the intensity and duration of exercise, muscle damage, the type of subjects, the method of examining gene expression, and the type of target tissue (tendon and muscle) can be effective. The effect mechanisms of sports training on decorin are complex and require more studies.
Demonstratively, the difference in the results of the studies can be attributed to the type of skeletal muscles (slow-twitch and fast-twitch) involved and the type of sports activity —no research related to the effect of different intensities of sports activity that simultaneously measured decorin levels. The study of Potter et al. (2017) examined the effect of exercise on TGF-β and decorin levels. Besides, the results indicated increased TGF-β levels in the training group and no change in decorin gene expression in the Achilles tendon of male Wistar rats (42). Sotoudeh et al. (2017) investigated the decorin gene expression in the soleus muscle as a result of eight weeks of intermittent aerobic exercise in an animal model of breast cancer. In addition, the study's results indicated an increase in decorin levels in the soleus muscle (2). This research's results are consistent with the results of the current research.
Skeletal muscle is an endocrine organ that not only contains essentialmetabolic molecules whose expression changes metabolism but also communicates with other tissues through the secretion of hormones known as myokines.
The co-activating factor of peroxisome proliferator-activated receptor-gamma (PGC1-a) is one of the crucial metabolic molecules expressed in muscle. It plays an essential role in maintaining glucose, lipid, and energy homeostasis, and its expression increases with exercise in skeletal muscle (43). Myokines are probably a bridge between the interaction between skeletal muscle and other tissues involved in homeostasis and energy metabolism, including fat tissue (8).
Therefore, regarding the expression of the physiological mechanism of the effect of exercise training on the expression of muslin, aplin, and decorin genes, seemingly, intervening factors such as the intensity and duration of exercise, muscle damage, the type of subjects, the method of examining gene expression, and the type of tissue, (tendon and muscle) can be the reason for the difference in the studies.
Conclusion
The present research is conducted in muslin, apelin, and decorin gene expression using rat samples with aerobic exercise, which is vital. In general, the results of this study showed that four weeks of moderate-intensity aerobic exercise significantly increased the gene expression of the studied variables. According to the present study's findings, performing aerobic exercise training by increasing the expression of musclin, apelin, and decorin plays an essential role in energy homeostasis, showing the importance of this type of exercise in health.
Acknowledgments
This article is taken from the thesis of a doctoral degree in exercise physiology student with the code of ethics IR.IAU.PIAU.REC.1401.009 in the Faculty of Physical Education, Islamic Azad University, Islamshahr Branch. The researchers express their utmost gratitude to the officials of the Islamic Azad University, Islamshahr branch, who helped us conduct this research with their participation, and to all the people who collaborated in this study.
Funding source
This study did not have any funds.Ethical statement
The Ethics Committee of the Islamic Azad University of Parand approved this study’s protocol (IR.IAU.PIAU.REC.1401.009).Conflict of interest
The research was self-funded, and the equipment necessary for conducting the study was provided by the Baqiyatallah University of Medical Sciences.Author contributions
Dr. Kazemzadeh, Dr Shirvani played pivotal roles in data collection, Statistical population collection and laboratory coordination. Their expertise and insights were crucial to the success of this research. Dr. Mirzayan Shanjani, Dr. Sedaghati played pivotal roles in setting up the background, statistical analysis of the research.
Type of Article: Original article |
Subject:
Health
Received: 2022/10/12 | Accepted: 2023/06/12 | Published: 2023/07/1
Received: 2022/10/12 | Accepted: 2023/06/12 | Published: 2023/07/1
References
1. Piccirillo R. Exercise-Induced Myokines With Therapeutic Potential for Muscle Wasting. Front Physiol. 2019;10:287. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Sotoodeh V, gharakhanloo R, khaligh fard S, khaligh fard S, alizadeh A. Protective effects of eight weeks interval aerobic exercise on decorin, TGF-β and tumor volume in atypical animal of breast cancer. jsmt. 2017;15(14):59-71.
3. Hoffmann C, Weigert C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb Perspect Med. 2017; 7(11): a029793. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Fathi M, Mohammadi A, Mir E, et al. Molecular structure of skeletal muscle and myokines. University of Applied Sciences: 1st Edition. Sabzeavar Beyhaq Publications. 2016.
5. Baskin KK, Winders BR, Olson EN. Muscle as a "mediator" of systemic metabolism. Cell Metab. 2015;21(2):237-48. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999; 277(6): E1130-41. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481(7382): 463-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Seldin MM, Wong GW. Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte. 2012;1(4):200-2. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017;216(1):149-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen D, Kolnes AJ, et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450(2):1089-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Guo A, Li K, Xiao Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets?. Exp Gerontol. 2020;139:111022. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9(11):2482-96. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Järvinen TA, Prince S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. Biomed Res Int. 2015;2015:654765. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Farahani SZ, Nazar Ali P, Rahmani H. The effect of high intensity interval training on Musclin and insulin in overweight female students [Master Thesis]. Tehran: Al-Zahra University; 2016.
16. Re Cecconi AD, Forti M, Chiappa M, Zhu Zh, Zingman LV, Cervo L, et al. Musclin, A Myokine Induced by Aerobic Exercise, Retards Muscle Atrophy During Cancer Cachexia in Mice. Cancers (Basel). 2019;11(10):1541. [View at Publisher] [DOI] [PMID] [Google scholar]
17. Röckl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60(3):145-53. [View at Publisher] [DOI] [PMID] [Google scholar]
18. Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond). 2014;38(5):707-13. [View at Publisher] [DOI] [PMID] [Google scholar]
19. Al-Jarrah M, Matalka I, Aseri HA, Mohtaseb A, Smirnova IV, Novikova L, et al. Exercise training prevents endometrial hyperplasia and biomarkers for endometrial cancer in rat model of type 1 diabetes. J Clin Med Res. 2010;2(5):207-14. [View at Publisher] [DOI] [PMID] [Google scholar]
20. Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337-46. [View at Publisher] [DOI] [PMID] [Google scholar]
21. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379-406. [view at publisher] [DOI] [PMID] [Google Scholar]
22. Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279(19):19391-5. [view at publisher] [DOI] [PMID] [Google Scholar]
23. Subbotina E, Sierra A, Zhu Z, Gao Z, Krishna Koganti SR, Reyes S, et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci USA. 2015;112(52):16042-7. [view at publisher] [DOI] [PMID] [Google Scholar]
24. Yu J, Zheng J, Liu XF, Feng ZL, Zhang XP, Cao LL, et al. Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz J Med Biol Res. 2016;49(5):e5129. [view at publisher] [DOI] [PMID] [Google Scholar]
25. Castan-Laurell I, Dray C, Knauf C, Cunduzova O, Valet Ph. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab. 2012;23(5):234-41. [view at publisher] [DOI] [PMID] [Google Scholar]
26. Kadoglou NP, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit. 2012;18(5):CR290-5. [view at publisher] [DOI] [PMID] [Google Scholar]
27. Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40(1):1-9. [view at publisher] [DOI] [PMID] [Google Scholar]
28. Bertrand C, Valet P, Castan-Laurell I. (2015). Apelin and energy metabolism. Front Physiol. 2015;6:115. [view at publisher] [DOI] [PMID] [Google Scholar]
29. He S, Li J, Wang J, Zhang Y. Hypoxia exposure alleviates impaired muscular metabolism, glucose tolerance, and aerobic capacity in apelin-knockout mice. FEBS Open Bio. 2019;9(3):498-509. [view at publisher] [DOI] [PMID] [Google Scholar]
30. Dray C, Sakar Y, Vinel C, Daviaud D, Masri B, Garrigues L, et al. The intestinal glucose-apelin cycle controls carbohydrate absorption in mice. Gastroenterology.2013;144(4):771-80. [view at publisher] [DOI] [PMID] [Google Scholar]
31. Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1):E59-67. [view at publisher] [DOI] [PMID] [Google Scholar]
32. Xu S, Han P, Huang M, Wu JC, Chang C, Tsao PS, et al. In vivo, ex vivo, and in vitro studies on apelin's effect on myocardial glucose uptake. Peptides. 2012;37(2):320-6. [view at publisher] [DOI] [PMID] [Google Scholar]
33. Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y, et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 2011;353(1-2):305-13. [view at publisher] [DOI] [PMID] [Google Scholar]
34. Fukaya M, Mizuno A, Arai H, Muto K, Uebanso T, Matsuo K, et al. Mechanism of rapid-phase insulin response to elevation of portal glucose concentration. Am. J. Physiol. Endocrinol. Metab. 2007;293(2):E515-22. [view at publisher] [DOI] [PMID] [Google Scholar]
35. Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab. 2010;36(4):257-62. [view at publisher] [DOI] [PMID] [Google Scholar]
36. Bekki M, Hashida R, Kawaguchi T, Goshima N, Yoshiyama T, Otsuka T, et al. The association between sarcopenia and decorin an exercise induced myokine in patients with chronic liver disease. JCSM Rap Com. 2018;1(2):1-10. [view at publisher] [DOI] [Google Scholar]
37. Kawaguchi T, Yoshio S, Sakamoto Y, Hashida R, Koya S, Hirota K, et al. Impact of decorin on the physical function and prognosis of patients with hepatocellular carcinoma. J Clin Med. 2020;9(4):936. [view at publisher] [DOI] [PMID] [Google Scholar]
38. Brandan E, Fuentes ME, Andrade W. The proteoglycan decorin is synthesized and secreted by differentiated myotubes. Eur J Cell Biol. 1991;55(2):209-16. [view at publisher] [PMID] [Google Scholar]
39. Xu SY, Liu SY, Xu L, Deng SY, He YB, Li SF, Ni GX. Response of decorin to different intensity readmill running. Mol Med Rep. 2018;17(6):7911-7. [view at publisher] [DOI] [PMID] [Google Scholar]
40. Potter RM, Huynh RT, Volper BD, Arthur KA, D'Lugos AC, Sørensen MA, Magnusson SP, Dickinson JM, Hale TM, Carroll CC. Impact of TGF-β inhibition during acute exercise on Achilles tendon extracellular matrix. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R157-64. [view at publisher] [DOI] [PMID] [Google Scholar]
41. Polyzos SA, Kountouras J, Shields K, Mantzoros CS. Irisin: a renaissance in metabolism? Metabolism. 2013;62(8):1037-44. [view at publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


