Volume 10, Issue 2 (5-2022)
Jorjani Biomed J 2022, 10(2): 10-23 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseinabadi F, Faraji T, Malmir M, Mohamadi H. Ameliorative Impact of Silymarin on the Male Reproductive System: An Updated Systematic Review. Jorjani Biomed J 2022; 10 (2) :10-23
URL: http://goums.ac.ir/jorjanijournal/article-1-873-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-873-en.html
1- Academic Center for Education, Culture and Research, Arak, Iran
2- Department of Midwifery, Tuyserkan Branch, Islamic Azad University, Tuyserkan, Iran , m.malmir66@hotmail.com
3- Department of Midwifery, Tuyserkan Branch, Islamic Azad University, Tuyserkan, Iran
2- Department of Midwifery, Tuyserkan Branch, Islamic Azad University, Tuyserkan, Iran , m.malmir66@hotmail.com
3- Department of Midwifery, Tuyserkan Branch, Islamic Azad University, Tuyserkan, Iran
Full-Text [PDF 881 kb]
(1288 Downloads)
| Abstract (HTML) (2749 Views)
One of the reasons for oxidative stress is the imbalance between ROS production and antioxidants (13, 14, 15). Oxidative stress is involved in many pathological conditions and diseases (16, 17, 18). Many studies have shown that there is a relationship between oxidative stress conditions in semen dysfunction (15, 19, 20, 21). Antioxidant mechanisms of silymarin in the reproductive tissue are likely to suppress ROS and protect gonadal cells and spermatozoa from getting damaged. Studies show silymarin can improve semen parameters as an antioxidant (1, 8, 9, 22, 23). Silymarin affects the enzyme systems associated with glutathione and superoxide dismutase and increases them, silymarin can increase the amount of cellular content of glutathione by increasing the substrate (cysteine) and inhibits lipid peroxidation (24, 25, 26) given that glutathione is the most abundant cellular antioxidant and be able to protect cells against the toxic effects of ROS (7) and also can inhibit lipid peroxidation (26). Moreover, silymarin can regulate membrane permeability and increase membrane stability in the presence of xenobiotic damage (22). Silymarin inhibits the uptake of toxins and prevents them from binding to the cell surface (27). Finally, silymarin can enter the nucleus and stimulate RNA polymerase enzymes, thereby increasing ribosome formation that, in turn, accelerates DNA and protein synthesis (25).
Oxidative stress is one of the important factors in male reproductive system disorders. Also, the most common way to deal with oxidative stress is to use antioxidants today. Hence, the present review intended to contribute a summary of prior research regarding silymarin antioxidant role in male reproduction disorder carried out.
Materials and Methods
Inclusion criteria in the present study included the evaluation of spermatogenesis, testicular tissue, blood hormones, and enzymes from Apr 1998 to Feb 2020. The electronic search was performed on the databases of WOS, PubMed, Science Direct, Scopus, EBSCO, and grey literature. Finally, the Google Scholar Database was used to ensure complete content and this study lasted from Jan 2021 to Jul 2021. The inclusion criteria of this study were contained andrological studies (spermatogenesis, spermiogenesis, and sperm parameters), histological and morphometrical studies (testicular tissue), and endocrinological studies (sex hormone). The present study was performed using the words MeSH including Silymarin, Milk thistle, Silybum marianum, testis, spermatogenesis, and sex hormones. The search results of the databases involved a total of 264 articles. The number of these articles was reduced to 78 after passing the identification, screening, and eligibility stages. Exclusion criteria included invalid journals, duplication, and lack of access to the full text (Fig 3). The steps related to searching for articles, results, and extracting data were performed independently by two researchers and in case of differences in each of the steps, they were reviewed by a third researcher. A search syntax was developed by using: Search ((((("silymarin"[MeSH Terms] OR silymarin [Text Word]) OR ("milk thistle"[MeSH Terms] OR Milk thistle [Text Word])) OR ("milk thistle"[MeSH Terms] OR Silybum marianum [Text Word])) AND ("testis" [MeSH Terms] OR testis [Text Word])) OR ("spermatogenesis"[MeSH Terms] OR Spermatogenesis [Text Word])) AND ("gonadal steroid hormones"[MeSH Terms] OR sex hormones [Text Word]).
.png)
Fig.3. Preferred Reporting Items for Systematic Analysis (PRISMA) diagram: the flow of information through the different phases of a Systematic Review
Silymarin acts as a powerful antioxidant.
This antioxidant protects the male reproductive system against various toxins that increase free radicals.
Silymarin is able to prevent lipid peroxidation and expression of pro-apoptotic genes in testicular tissue.
Full-Text: (883 Views)
Highlights
Introduction
Silybum marianum is widely used in the treatment of many disorders (Fig 1) (1). Silymarin (C25H22O10), a flavonoid and polyphenolic molecule, is extracted from the seeds of Silybum marianum (Fig 2) (1, 2, 3). Silymarin can be effective in many including infertility in men and women (4). In addition to having other biological properties, these compounds are recognized as antioxidants (5, 6). In 1959, silybin was discovered as the first member of a new family of natural compounds called flavonolignans and was known as the active and dominant compound in silymarin (1, 7, 8). Many studies have shown the protective and antioxidant properties of silymarin against the side effects of chemotherapy drugs and environmental toxins on sperm (9-12).

Fig.1. The specie of Silybum Marianum
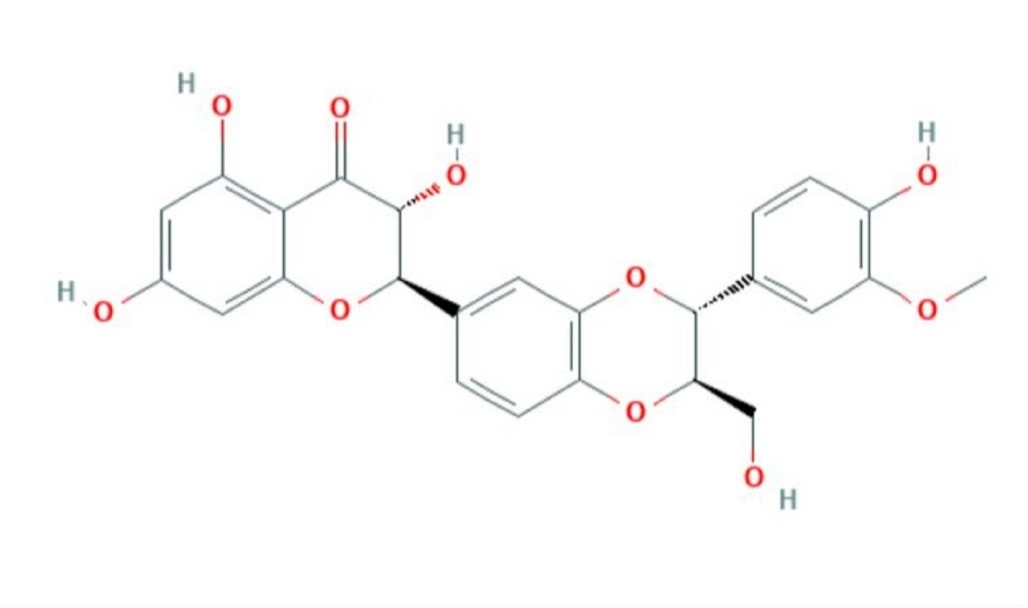
Fig.2. Chemical Structure Depiction (C25H22O10) of silymarin
- Silymarin acts as a powerful antioxidant.
- This antioxidant protects the male reproductive system against various toxins that increase free radicals.
- Silymarin is able to prevent lipid peroxidation and expression of pro-apoptotic genes in testicular tissue.
Introduction
Silybum marianum is widely used in the treatment of many disorders (Fig 1) (1). Silymarin (C25H22O10), a flavonoid and polyphenolic molecule, is extracted from the seeds of Silybum marianum (Fig 2) (1, 2, 3). Silymarin can be effective in many including infertility in men and women (4). In addition to having other biological properties, these compounds are recognized as antioxidants (5, 6). In 1959, silybin was discovered as the first member of a new family of natural compounds called flavonolignans and was known as the active and dominant compound in silymarin (1, 7, 8). Many studies have shown the protective and antioxidant properties of silymarin against the side effects of chemotherapy drugs and environmental toxins on sperm (9-12).

Fig.1. The specie of Silybum Marianum

Fig.2. Chemical Structure Depiction (C25H22O10) of silymarin
One of the reasons for oxidative stress is the imbalance between ROS production and antioxidants (13, 14, 15). Oxidative stress is involved in many pathological conditions and diseases (16, 17, 18). Many studies have shown that there is a relationship between oxidative stress conditions in semen dysfunction (15, 19, 20, 21). Antioxidant mechanisms of silymarin in the reproductive tissue are likely to suppress ROS and protect gonadal cells and spermatozoa from getting damaged. Studies show silymarin can improve semen parameters as an antioxidant (1, 8, 9, 22, 23). Silymarin affects the enzyme systems associated with glutathione and superoxide dismutase and increases them, silymarin can increase the amount of cellular content of glutathione by increasing the substrate (cysteine) and inhibits lipid peroxidation (24, 25, 26) given that glutathione is the most abundant cellular antioxidant and be able to protect cells against the toxic effects of ROS (7) and also can inhibit lipid peroxidation (26). Moreover, silymarin can regulate membrane permeability and increase membrane stability in the presence of xenobiotic damage (22). Silymarin inhibits the uptake of toxins and prevents them from binding to the cell surface (27). Finally, silymarin can enter the nucleus and stimulate RNA polymerase enzymes, thereby increasing ribosome formation that, in turn, accelerates DNA and protein synthesis (25).
Oxidative stress is one of the important factors in male reproductive system disorders. Also, the most common way to deal with oxidative stress is to use antioxidants today. Hence, the present review intended to contribute a summary of prior research regarding silymarin antioxidant role in male reproduction disorder carried out.
Materials and Methods
Inclusion criteria in the present study included the evaluation of spermatogenesis, testicular tissue, blood hormones, and enzymes from Apr 1998 to Feb 2020. The electronic search was performed on the databases of WOS, PubMed, Science Direct, Scopus, EBSCO, and grey literature. Finally, the Google Scholar Database was used to ensure complete content and this study lasted from Jan 2021 to Jul 2021. The inclusion criteria of this study were contained andrological studies (spermatogenesis, spermiogenesis, and sperm parameters), histological and morphometrical studies (testicular tissue), and endocrinological studies (sex hormone). The present study was performed using the words MeSH including Silymarin, Milk thistle, Silybum marianum, testis, spermatogenesis, and sex hormones. The search results of the databases involved a total of 264 articles. The number of these articles was reduced to 78 after passing the identification, screening, and eligibility stages. Exclusion criteria included invalid journals, duplication, and lack of access to the full text (Fig 3). The steps related to searching for articles, results, and extracting data were performed independently by two researchers and in case of differences in each of the steps, they were reviewed by a third researcher. A search syntax was developed by using: Search ((((("silymarin"[MeSH Terms] OR silymarin [Text Word]) OR ("milk thistle"[MeSH Terms] OR Milk thistle [Text Word])) OR ("milk thistle"[MeSH Terms] OR Silybum marianum [Text Word])) AND ("testis" [MeSH Terms] OR testis [Text Word])) OR ("spermatogenesis"[MeSH Terms] OR Spermatogenesis [Text Word])) AND ("gonadal steroid hormones"[MeSH Terms] OR sex hormones [Text Word]).
.png)
Fig.3. Preferred Reporting Items for Systematic Analysis (PRISMA) diagram: the flow of information through the different phases of a Systematic Review
Results
Increased ROS during oxidative stress is known to impair oxidant-antioxidant balance and contribute to the peroxidation of unsaturated fatty acids in the sperm membrane and disorder in spermatogenesis and sperm function (16, 17, 31). The quantity and quality of spermatogenesis are determined based on the evaluation of parameters such as count, motility, viability, DNA damage, normal morphology, and population of Sertoli and spermatogenic cells (32-34). Numerous studies have shown that silymarin compensates for the integrity of plasma membranes and acrosomes, motility, viability, and sperm DNA fragmentation (4, 30, 35-38).
Malondialdehyde (MDA) levels are considered an indicator of lipid peroxidase activity and the end product of lipid peroxidation (39). Studies have shown that silymarin increases total antioxidant capacity and decrease MDA (4, 36, 40, 41). Silymarin due to its antioxidant properties can prevent lipid peroxidation of cell membranes and maintain sperm membrane integrity (26).
Silymarin can also increase testosterone levels, which in turn promotes sperm health and maintains cell division (3, 42). It has been shown that silibinin may improve germinal epithelium function and spermatogenesis by preventing oxidative stress (3).
Moreover, silymarin as a potent antioxidant can eliminate free radicals including ROS, and subsequently reduce DNA fragmentation (1, 14). Research has shown that silymarin increases Bcl2 gene expression and decreases Bax and caspase 3 expression, thereby reducing apoptosis in testicular tissue (43).
Transcription factor E2F1 is one of the factors involved in the apoptosis, count, and viability of sperm. Silymarin can reduce E2F1 expression and thus prevents overstimulation of apoptosis in sperm cells (41) (Table 1).
Sperm are vulnerable to ROS at different stages of spermatogenesis (25). Several internal and external factors can interact with lipids and proteins to cause oxidative stress and ROS production following that occur lipid peroxidation, DNA fragmentation, raise superoxide ions in mitochondria, decreased antioxidant activity, and ultimately spermatogenesis disorder (46).
Numerous studies have shown that low testosterone levels reduce testicular function (32). Moreover, increased apoptosis is one of the results of oxidative stress that reduces motility and count of sperm (25, 46). Silymarin increases testicular weight, epithelial height, spermatogenesis, and total antioxidant capacity, and decreases MDA, apoptosis, and edema (3, 47-53).
Numerous studies have shown that silymarin is a powerful antioxidant that can protect testicular tissue from the adverse effects of oxidative stress (3, 54). Table 2 evaluates and compares the effects of silymarin on testicular tissue (Table 2).
Many studies have illustrated that silymarin can increase LH, FSH, and GnRH hormones (43, 47, 55-57). Testosterone is important in the starting and continuing of spermatogenesis and its reduction contributes to defects in spermatogenesis (58). Silymarin can also increase testosterone levels, which in turn increases spermatogenesis (47, 56, 59). Moreover, silymarin can regulate serum levels of LH, FSH, inhibin B, and testosterone against toxins, and vis-a-vis can reduce lipid peroxidation and MDA (60-62).
Increased ROS during oxidative stress is known to impair oxidant-antioxidant balance and contribute to the peroxidation of unsaturated fatty acids in the sperm membrane and disorder in spermatogenesis and sperm function (16, 17, 31). The quantity and quality of spermatogenesis are determined based on the evaluation of parameters such as count, motility, viability, DNA damage, normal morphology, and population of Sertoli and spermatogenic cells (32-34). Numerous studies have shown that silymarin compensates for the integrity of plasma membranes and acrosomes, motility, viability, and sperm DNA fragmentation (4, 30, 35-38).
Malondialdehyde (MDA) levels are considered an indicator of lipid peroxidase activity and the end product of lipid peroxidation (39). Studies have shown that silymarin increases total antioxidant capacity and decrease MDA (4, 36, 40, 41). Silymarin due to its antioxidant properties can prevent lipid peroxidation of cell membranes and maintain sperm membrane integrity (26).
Silymarin can also increase testosterone levels, which in turn promotes sperm health and maintains cell division (3, 42). It has been shown that silibinin may improve germinal epithelium function and spermatogenesis by preventing oxidative stress (3).
Moreover, silymarin as a potent antioxidant can eliminate free radicals including ROS, and subsequently reduce DNA fragmentation (1, 14). Research has shown that silymarin increases Bcl2 gene expression and decreases Bax and caspase 3 expression, thereby reducing apoptosis in testicular tissue (43).
Transcription factor E2F1 is one of the factors involved in the apoptosis, count, and viability of sperm. Silymarin can reduce E2F1 expression and thus prevents overstimulation of apoptosis in sperm cells (41) (Table 1).
Sperm are vulnerable to ROS at different stages of spermatogenesis (25). Several internal and external factors can interact with lipids and proteins to cause oxidative stress and ROS production following that occur lipid peroxidation, DNA fragmentation, raise superoxide ions in mitochondria, decreased antioxidant activity, and ultimately spermatogenesis disorder (46).
Numerous studies have shown that low testosterone levels reduce testicular function (32). Moreover, increased apoptosis is one of the results of oxidative stress that reduces motility and count of sperm (25, 46). Silymarin increases testicular weight, epithelial height, spermatogenesis, and total antioxidant capacity, and decreases MDA, apoptosis, and edema (3, 47-53).
Numerous studies have shown that silymarin is a powerful antioxidant that can protect testicular tissue from the adverse effects of oxidative stress (3, 54). Table 2 evaluates and compares the effects of silymarin on testicular tissue (Table 2).
Many studies have illustrated that silymarin can increase LH, FSH, and GnRH hormones (43, 47, 55-57). Testosterone is important in the starting and continuing of spermatogenesis and its reduction contributes to defects in spermatogenesis (58). Silymarin can also increase testosterone levels, which in turn increases spermatogenesis (47, 56, 59). Moreover, silymarin can regulate serum levels of LH, FSH, inhibin B, and testosterone against toxins, and vis-a-vis can reduce lipid peroxidation and MDA (60-62).
Table 1. Evaluation of the effect of silymarin on men and different species of animals (Spermatogenesis)
| Author, year (ref) | Species | Type of response | ||||||
| Dose of SM & Duration of treatment |
Mot | Abn | Cou | Via | Other Parameters | T & D | ||
| Aghashahi et al., 2020 (9) | Human | 1 μM – 180 min in vitro |
↑ | ↑ | ↑ Acrosome and plasma membrane integrity, ↑ total antioxidant capacity, ↓ MDA |
Aluminum | ||
| Aghashahi et al., 2020 (9) | Human | 1 μM – 180 min in vitro |
↑ | ↑ | ↑ Acrosome and plasma membrane integrity, ↑ TAC, ↓ MDA |
|||
| Etemadi et al., 2020 (10) | Human | 2 μM- 180 min in vitro |
↓ SDF, ↑ nucleus diameter, ↑ MMP |
Cadmium | ||||
| Rahimi-Madiseh et al., 2020 (11) | NMRI mice | 100 and 200 mg/kg – 28 days | ↑ | ↓ | ↑ | ↑ | Nicotine | |
| Fatehi et al., 2017 (30) | NMRI mice | 50 mg/kg – 7 days | ↑ | ↓ | ↑ | ↑ | ↓ Immotile sperm, ↑ Progressive motility, ↑ testes weight, ↓ SDF |
|
| Fatehi et al., 2017 (30) | NMRI mice | 50 mg/kg – 7 days | ↑ | ↓ | ↑ | ↑ | ↓ SDF | γ-ray |
| Ali Mehr & Parisoush, 2016 (35) | Taleshi ram | 0, 50, 100, 150 and 200 µg/ml – 72 hours in vitro | ↑ | ↑ | ↑ Acrosome and plasma membrane integrity, ↓ MDA |
|||
| Ziaeirad et al., 2015 (36) | Rooster | 100 μg/mL– 48, 72 hours | ↑ | ↑ | ↓ MDA | |||
| Eskandari et al., 2017 (37) | Ram | 20 µM – 180 min in vitro |
↓ SDF | Sodium arsenite | ||||
| Eskandari et al., 2016 (38) | Ram | 20 µM – 180 min | ↑ Acrosome integrity | Sodium arsenite | ||||
| Shafiei-Roudbari et al., 2017 (41) | Wistar rats | 50 mg/kg – 10 days | ↑ | ↓ | ↑ | ↓ Sperm DNA fragmentation, ↓ nitric oxide, ↑ TAC |
Doxorubicin | |
| Heidari Khoei et al., 2018 (43) | Wistar rats | 100, 200 mg/kg - 5 weeks |
↑ | ↑ | ↑ | ↓ SDF, ↑ Normal sperm morphology (non-significant) | Diabetic | |
| Abo El-atta et al.,2020 (47) | SD rat | 200 mg/kg – 30 days | ↑ | ↑ | ↑ Spermatogenesis, ↓ Abnormal morphology (%) |
Malathion | ||
| Hamid et al., 2016 (48) | Albino rat | 150 mg/kg - 35 days | ↑ | ↑ | ↑ Testis weight | CCl4 | ||
| Yaman et al., 2018 (50) | Wistar albino rats | 200 mg/kg – 6 weeks | ↑ | ↓ | ↑ | ↓ MDA, ↑ Spermatogenesis |
Methotrexate | |
| Khalil, 2002 (51) | Albino rat | 151.2 mg/kg – 1 month | ↑ Spermatogenesis | |||||
| Chen et al., 2015 (53) | ICR mice | 5, 10, 20 mg/mL – 120 min in vitro |
↓ | ↓ Glucose-activated sperm motility, ↓ VAP & VCL |
High glucose (HG) | |||
| Abedi et al., 2016 (55) | Wistar rats | 150 mg/kg – 28 days | ↑ Spermatids, ↑ spermatozoa cells |
|||||
| Attia et al., 2017 (56) | Rabbit bucks | 5 and 10 g/kg – 8 weeks | ↑ | ↑ | ↑ Sperm concentration, ↑ total sperm output |
|||
| Al-Moziel MS et al., 2020 (57) | Albino rat | 100 mg/kg – 30 days | ↑ | ↓ | ↑ | ↑ | ↑ Spermatogenesis | Tadalafil |
| Sahreen et al., 2013 (59) | SD rat | 50 mg/kg – twice a week for eight weeks | ↓ SDF | CCl4 | ||||
| Malekinejad et al., 2012 (61) | Wistar rat | 50 mg/kg – 4 weeks | ↓ SDF, ↓ carbonyl stress | Doxorubicin | ||||
| Eskandari et al., 2016 (66) | Ram | 20 µM – 180 min in vitro |
↑ | ↑ | ↑ Intact mitochondrial membrane |
Sodium arsenite | ||
| Choobineh et al., 2018 (67) | Ram | 0.05, 0.1 and 0.15 mM –180 min in vitro |
↓ SDF, ↓ apoptosis |
lithium chloride | ||||
| Choobineh et al., 2018 (68) | Ram | 0.1 and 0.15 mM – 180 min in vitro |
↑ | ↑ Acrosome integrity | lithium chloride | |||
| Momeni et al., 2018 (69) | Ram | 0.5 μM – 180 min in vitro |
↑ | ↑ | ↑ Intact mitochondrial membrane | Aluminum | ||
| Momeni et al., 2015 (70) | Ram | 0.5μM – 180 min in vitro |
↑ Sperm plasma membrane integrity, ↑ sperm acrosome integrity |
Aluminum Chloride | ||||
Anderson et al., 1998 (72) |
Human | 100, 200, 300, and 500 µM | ↓ DNA damage | Trp-P-2 & IQ | ||||
| Zahra Z et al., 2020 (74) | SD rat | 200 mg/kg – 30 days | ↑ | ↓ | ↑ | ↑ | ↓ DNA damage | BPA |
| El-Sheshtawy et al., 2017 (77) | Bull | 0.18, 0.36, 0.54 and 0.72 mg/ml in vitro | ↑ | ↓ | ↑ | ↑ Intact spermatozoa, membranes | Cryopreservation | |
SM: Silymarin; NIMRI: Naval Medical Research Institute; SD: Sprague-Dawley; Mot: Motility; Abn: Abnormality; Cou: Count; Via: Viability; T&D: Against Toxin & Diseases; SDF: Sperm DNA fragmentation; BPA: Bisphenol A; TAC: Total antioxidant capacity; MDA: Malondialdehyde; MMP: Mitochondrial Membrane Potential; Trp-P-2 & IQ; 3-amino-1-methyl-5H-pyrido (4,3-b) indole& 2-amino-3-methylimidazo-(4,5-f) quinolone; VAP: average pathway velocity; VCL: curvilinear velocity; ↑: Increase or Improve; ↓: Decrease, (Comparison in the toxin/disease group with Silymarin + toxin/disease group).
Table 2. Evaluation of the effect of silymarin on men and different species of animals (testicular tissue)
Table 2. Evaluation of the effect of silymarin on men and different species of animals (testicular tissue)
| Author, year (ref) | Species | Type of Response | |||
| Dose of SM & Duration of treatment |
T & D | Oxidative stress & Apoptosis |
Histology, Testicular biochemistry & PCR |
||
| Moshtaghion et al., 2013 (40) | Wistar rat | 50 mg/kg - 42 days | Varicocele | ↓ Ap, ↓ OS | ↓ MDA, Maintain TTM, ↓ E2f1, ↓ dissociated germinal epithelium in the seminiferous tubules, ↑ negative TDI ↓ edema of connective tissue, ↑ Number of Leydig cells&Sertoli cells, ↑ percentage of seminiferous tubules and spermiogenesis indices |
| Shafiei-Roudbari et al., 2017 (41) | Wistar rats | 50 mg/kg – 10 days | Doxorubicin | ↓ Ap, ↓ OS | ↓ NO, ↓ E2F1 expression, ↑ TAC |
| Heidari Khoei et al., 2018 (43) | Wistar rats | 100, 200 mg/kg – 5 wk | Diabetic | ↓ Ap, ↓ OS | ↓ MDA, ↑ SOD, ↑ CAT (non-significant), ↓ LPO ↑ Bcl-2, ↓ Bax, ↓ Caspase-3 |
| Abo El-atta et al.,2020 (47) | SD rat | 200 mg/kg – 30 days | Malathion | ↑ Spermatogenesis, ↓ necrotic seminiferous tubules, ↑ Testicular weight | |
| Hamid et al., 2018 (48) | Albino rat | 150 mg/kg – 35 days |
CCl4 | ↓ OS | ↑ Testis weight, the tail of the sperm are thin and the sperm offspring are similar to the normal state |
| Sheweita SA et al., 2016 (49) | Rat | 25 mg/kg –1 wk | B[a]P | ↑ CAT, ↑ GPx, ↑ SOD, ↓ TBARS, ↓ edema, ↓ necrotic seminiferous tubule, ↑ 17 HSD activity | |
| Yaman et al., 2018 (50) | Wistar albino rats |
200 mg/kg – 6 wks |
Methotrexate | ↓ OS | ↓ Atrophy & degeneration of germinal cells, ↑ Spermatogenesis, ↓ pathomorphological changes in the spermatogonia, ↓ interstitial edema, ↑ SOD2, ↑ GPx1, ↑ CAT |
| Yaman et al., 2018 (50) | Wistar albino rats |
200 mg/kg – 6 wks |
↓ OS | ↑ GPx1, ↑ CAT, ↓ MDA | |
| Khalil, 2002 (51) | Albino rat | 151.2 mg/kg – 1 month |
↑ Spermatogenesis | ||
| Çeribas et al., 2020 (52) | Japanese quail chicks | Diet including 1% MTS – 35 days | High energy diets (HED) | ↓ OS | ↑ Round spermatid & Elongated spermatid ↑ sperm count, ↓ MDA |
| El-Hady A et al., 2015 (54) | Albino rat | 18 mg/Kg - 3 times/week for 2 months | Mobile phone radiation | ↓ OS | ↑ Number of Leydig cells, ↑ seminiferous tubules diameter, ↑ total protein, ↑ DNA content of the nuclei of spermatogenic cells and Leydig cells, ↓ collagen fibers |
| Sahreen et al., 2013 (59) | SD rat | 50 mg/kg – twice a week for 8 wk | CCl4 | ↓ OS | ↓ TBARS & H2O2, ↑ SOD, ↑ CAT ↑ GSH, ↑ GST, ↑ GPx, ↓ abnormality of germinative epithelium, ↓ sperm with abnormal shape |
| Faraji et al., 2018 (60) | NMRI mice | 100 mg/kg – 24 hr |
Cadmium | ↓ OS | ↑ TAC, ↑ SOD, ↑ CAT, ↑ GPx, ↓ MDA, ↑ the testis diameter, wall thickness of the seminiferous tubules, and nucleus diameter of spermatogonia |
| Malekinejad et al., 2012 (61) | Wistar rat | 50 mg/kg– 4 wk | Doxorubicin | ↓ OS | ↓ Interstitial edema, ↑ Depletion of the seminiferous tubules, ↓ c-myc expression |
| Rafiee et al., 2016 (62) | Wistar rat | 50 mg/kg – 28 days | CCl4 | ↓ OS | ↑ Spermiogenesis index, ↑ absolute testis weight, ↑ seminiferous tubules diameter, ↓ MDA, ↑ CAT, ↑ the thickness of the epithelium |
| Ali Shah et al., 2017 (71) | SD rat | 200 mg/kg – 60 days | CCl4 | ↓ OS | ↑ SOD, ↑ CAT, ↑ POD, ↑ GSH, ↑ GST, ↑ GPx, ↓ TBARS, ↑ the morphology of the seminiferous tubules & the density of germ cells |
| Zahra Z et al., 2020 (74) | SD rat | 200 mg/kg – 30 days | BPA | ↓ OS | ↑ Seminiferous tubules diameter, ↑ testis weight, ↑ GSH, ↑ CAT, ↑ SOD, ↓ TBARS & H2O2, ↓ ROS |
| Sajedianfard et al., 2016 (75) | Wistar rat | 175 mg/kg – 14 days | Busulfan | ↓ OS | ↑ SOD, ↑ GPX, ↓ MDA |
| Kashif Saleemi et al., 2019 (76) | Japanese quails | 250 mg/kg – 60 days | Cadmium | ↑ Spermatogenesis, ↑ testis volume, ↑ testis weight | |
| Marzban et al., 2017 (78) | SD rat | 100, 200 mg/kg – 24 hr | γ-ray | ↓ Ap |
↑ Tube diameter, ↑ the height of seminiferous epithelium, ↑ number of spermatogonia, primary spermatocyte, round spermatid, spermatozoa, ↓ Leydig cell apoptosis |
| Chen et al., 2019 (79) | SD rat |
150 mg/ kg – 20 wk | AGE | ↑ Number of epididymal sperm, ↓ abnormal sperm rate, ↓ MDA | |
SM: Silymarin; NIMRI: Naval Medical Research Institute; SD: Sprague-Dawley; Ap: Apoptosis; OS: Oxidative Stress; CAT: Catalase; SOD: Superoxide dismutase; BPA: Bisphenol A; B[a]P: benzo[a]pyrene; LPO: Lipid peroxidation; TAC: Total antioxidant capacity; GSH-Px: Glutathione peroxidase; MDA: Malondialdehyde; GST: Glutathione S Transferase; ROS: Reactive oxygen species; T & D: Against Toxin & Diseases; AGE: Advanced glycation end products; TBARS: Thiobarbituric Acid Reactive Substances; H2O2: Hydrogen peroxide; HSD: 17-β hydroxysteroid dehydrogenase; NO: Nitric oxide; TDI: tubular differentiation index; Gpx: Glutathione peroxidase; ↑: Increase or Improve; ↓: Decrease, (Comparison in the toxin/disease group with Silymarin + toxin/disease group).
Table 3. Evaluation of the effect of silymarin on men and different species of animals (Endocrinology and Blood biochemistry)
Table 3. Evaluation of the effect of silymarin on men and different species of animals (Endocrinology and Blood biochemistry)
| Author, year (ref) | Species | Type of Response | |||
| Dose of SM & Duration of treatment |
Endocrinology | Blood biochemistry | T & D | ||
| Heidari Khoei et al., 2018 (43) | Wistar rats | 100 and 200 mg/kg - 5 wk |
↑ T | Diabetic | |
| Abo El-atta et al.,2020 (47) | SD rat | 200 mg/kg – 30 days | ↑ FSH, ↑ LH | ||
| Abo El-atta et al.,2020 (47) | SD rat | 200 mg/kg – 30 days | ↑ FSH, ↑ LH, ↑ T | ↑ BuChE | Malathion |
| Khalil, 2002 (51) | Albino rat | 151.2 mg/kg – 1 month |
↑ T, ↑ LH, estradiol not changed | ||
| Abedi et al., 2016 (55) | Wistar rat | 150 mg/kg – 28 days | ↑ FSH, ↑ LH, ↑ T, ↑ GnRH | ||
| Attia et al., 2017 (56) | Rabbit bucks | 5 and 10 g/kg - 8 wk | ↑ T | ↓ ALT, ↑ TAC (non-significant) | |
| Al-Moziel MS et al., 2020 (57) | Albino rat | 100 mg/kg – 30 days | ↑ FSH, ↑ LH, ↑ T | Tadalafil | |
| Sahreen et al., 2013 (59) | SD rat | 50 mg/kg – twice a week for 8 wk | ↑ FSH, ↑ LH, ↑ T, ↓ Prolactin, ↓ Estradiol | ↑ SOD, ↑ CAT, ↑ GSH, ↑ GST, ↑ GPx | CCl4 |
| Faraji et al., 2018 (60) | NMRI mice | 100 mg/kg – 24 hrs | ↑ T | ↓ MDA | Cadmium chloride |
| Malekinejad et al., 2012 (61) | Wistar rat | 50 mg/kg – 4 wk | ↑ FSH, ↑ LH, ↑ T, ↑ IB | Doxorubicin | |
| Rafiee et al., 2016 (62) | Wistar rat | 50 mg/kg – 28 days | ↑ T | CCl4 | |
| Ali Shah et al., 2017 (71) | SD rat | 200 mg/kg – 60 days | ↑ T | CCl4 | |
| Zahra Z et al., 2020 (74) | SD rat | 200 mg/kg – 30 days | ↑ FSH, ↑ LH, ↑ T | BPA | |
SM: Silymarin; NIMRI: Naval Medical Research Institute; SD: Sprague-Dawley; T: Testosterone; MDA: Malondialdehyde; CAT: Catalase; GSH-Px: Glutathione peroxidase; SOD: Superoxide dismutase; TAC: Total antioxidant capacity; LH: Luteinizing hormone; GnRH: Gonadotropin-releasing hormone; FSH: Follicle-stimulating hormone; ALT: Alanine aminotransferase activity; BPA: Bisphenol A; IB: Inhibin B; BuChE: butyryl Cholinesterase; ↑: Increase or Improve; ↓: Decrease; T&D: Against Toxin & Diseases, (Comparison in the toxin/disease group with Silimarin + toxin/disease group).
Discussion
The present study was performed to investigate the antioxidant effect of silymarin in the male reproductive system that has been exposed to toxins and environmental pollutants.
Spermatogenesis is a coordinated, orderly, long, and complex process that takes place in the germinal epithelium (28, 29). On the Other hand, seminiferous tubules are very sensitive to endogenous and exogenous stresses and exposure of the testicle to such conditions affects the somatic cells or germ cells at various stages of differentiation and leads to temporary or permanent irreversible infertility (30).
Many studies based on human and animal exposure to environmental toxins showed the negative impact of toxins on sperm quality and quantity (63). These toxins may also damage the DNA of sperm (63-65). Although silymarin therapy has an effective role in the improvement of sperm-related parameters and fertility against various toxins (68, 69, 71, 72). Research has shown that silymarin increases the concentration of norepinephrine. Norepinephrine is one of the factors that could influence the hypothalamus-pituitary-testis axis, and it increases GnRH and gonadotropins (LH and FSH hormones) through the synthesis of nitric oxide (48, 55). There is a relationship between the concentration of LH and the number of spermatogenic cells (48). LH by binding to Leydig cell increases the secretion of testosterone. Testosterone is an important factor in the spermatogenesis process (55). Also, FSH by binding to the Sertoli cell able to increase the concentration of ABP (androgen binding protein) and ABP can increase the concentration of testosterone in the seminiferous tubule to promote spermatogenesis (8, 55). On the other hand, silymarin can illustrate its antioxidant properties in 5 ways, such as 1. Direct scavenging of free radicals, 2. Preventing the formation of free radicals by inhibiting the specific enzymes responsible for their production of them, or by maintaining the integrity of the mitochondrial electron transfer chain, 3. by participating in maintaining the optimal redox conditions (oxidation and reduction) of the cell by activating a range of antioxidant enzymes and non-enzymatic antioxidants, 4. Activation of protective genes, responsible for the synthesis of protective molecules, including HSP (Heat shock proteins) and thioredoxin, 5. Reducing inflammatory responses by inhibiting NF-κB (Nuclear Factor-κB) pathways (22, 23).
Silymarin with its antioxidant effect can counteract the effects of various toxins such as sodium arsenite (66), lithium chloride (67, 68), aluminum (69, 70), tetrachloride Carbon (48, 71), Trp-P-2 and IQ (72), malathion (47), doxorubicin (61), acetate (73), methotrexate (50), nicotine (6), bisphenol A (74), busulfan (75) and cadmium (5, 60, 76). Silymarin also exerts similar beneficial effects on the side effects of diseases such as diabetes and varicocele (40, 43).
Spermatogenesis is a dynamic and controlled process that involves spermatogonia proliferation, meiotic divisions of spermatocytes, and differentiation of spermatids into sperm (44).
Sertoli cells and the height of the germinal epithelium regulate spermatogenesis by providing structural and nutritional support to the germ cells. Sertoli cells also control germ cell populations through apoptotic pathways (39, 45). In addition to the physiological apoptosis of germ cells that occurs continuously throughout life, external disorders such as radiation or exposure to toxic substances increase the apoptosis (39).
Besides, silymarin can be considered a promising plant protection agent in complementary medicine that may act an important role in protecting spermatocytes against the potential effects of freezing damage and γ-rays (30, 77, 78).
Conclusion
The results of studies show that antioxidants in the male reproductive system reduce oxidative stress in the testis and improve spermatogenesis. Many studies have shown the protective and antioxidant properties of silymarin against damage from chemotherapeutic drugs and environmental toxins in sperm. Also, the main published studies show the positive effects of silymarin on increasing the quality and quantity of sperm. Therefore, it is recommended that silymarin be prescribed to treat diseases caused by the effects of oxidative stress on the male reproductive system and improve fertility.
Acknowledgments
The authors of this article consider it necessary to appreciate the efforts of Hamideh Khodabandeh Lou and Razieh Bayat.
Conflicts of interest
There is no conflict of interest.
The present study was performed to investigate the antioxidant effect of silymarin in the male reproductive system that has been exposed to toxins and environmental pollutants.
Spermatogenesis is a coordinated, orderly, long, and complex process that takes place in the germinal epithelium (28, 29). On the Other hand, seminiferous tubules are very sensitive to endogenous and exogenous stresses and exposure of the testicle to such conditions affects the somatic cells or germ cells at various stages of differentiation and leads to temporary or permanent irreversible infertility (30).
Many studies based on human and animal exposure to environmental toxins showed the negative impact of toxins on sperm quality and quantity (63). These toxins may also damage the DNA of sperm (63-65). Although silymarin therapy has an effective role in the improvement of sperm-related parameters and fertility against various toxins (68, 69, 71, 72). Research has shown that silymarin increases the concentration of norepinephrine. Norepinephrine is one of the factors that could influence the hypothalamus-pituitary-testis axis, and it increases GnRH and gonadotropins (LH and FSH hormones) through the synthesis of nitric oxide (48, 55). There is a relationship between the concentration of LH and the number of spermatogenic cells (48). LH by binding to Leydig cell increases the secretion of testosterone. Testosterone is an important factor in the spermatogenesis process (55). Also, FSH by binding to the Sertoli cell able to increase the concentration of ABP (androgen binding protein) and ABP can increase the concentration of testosterone in the seminiferous tubule to promote spermatogenesis (8, 55). On the other hand, silymarin can illustrate its antioxidant properties in 5 ways, such as 1. Direct scavenging of free radicals, 2. Preventing the formation of free radicals by inhibiting the specific enzymes responsible for their production of them, or by maintaining the integrity of the mitochondrial electron transfer chain, 3. by participating in maintaining the optimal redox conditions (oxidation and reduction) of the cell by activating a range of antioxidant enzymes and non-enzymatic antioxidants, 4. Activation of protective genes, responsible for the synthesis of protective molecules, including HSP (Heat shock proteins) and thioredoxin, 5. Reducing inflammatory responses by inhibiting NF-κB (Nuclear Factor-κB) pathways (22, 23).
Silymarin with its antioxidant effect can counteract the effects of various toxins such as sodium arsenite (66), lithium chloride (67, 68), aluminum (69, 70), tetrachloride Carbon (48, 71), Trp-P-2 and IQ (72), malathion (47), doxorubicin (61), acetate (73), methotrexate (50), nicotine (6), bisphenol A (74), busulfan (75) and cadmium (5, 60, 76). Silymarin also exerts similar beneficial effects on the side effects of diseases such as diabetes and varicocele (40, 43).
Spermatogenesis is a dynamic and controlled process that involves spermatogonia proliferation, meiotic divisions of spermatocytes, and differentiation of spermatids into sperm (44).
Sertoli cells and the height of the germinal epithelium regulate spermatogenesis by providing structural and nutritional support to the germ cells. Sertoli cells also control germ cell populations through apoptotic pathways (39, 45). In addition to the physiological apoptosis of germ cells that occurs continuously throughout life, external disorders such as radiation or exposure to toxic substances increase the apoptosis (39).
Besides, silymarin can be considered a promising plant protection agent in complementary medicine that may act an important role in protecting spermatocytes against the potential effects of freezing damage and γ-rays (30, 77, 78).
Conclusion
The results of studies show that antioxidants in the male reproductive system reduce oxidative stress in the testis and improve spermatogenesis. Many studies have shown the protective and antioxidant properties of silymarin against damage from chemotherapeutic drugs and environmental toxins in sperm. Also, the main published studies show the positive effects of silymarin on increasing the quality and quantity of sperm. Therefore, it is recommended that silymarin be prescribed to treat diseases caused by the effects of oxidative stress on the male reproductive system and improve fertility.
Acknowledgments
The authors of this article consider it necessary to appreciate the efforts of Hamideh Khodabandeh Lou and Razieh Bayat.
Conflicts of interest
There is no conflict of interest.
Type of Article: Review Article |
Subject:
Health
Received: 2021/12/11 | Accepted: 2022/04/18 | Published: 2022/05/28
Received: 2021/12/11 | Accepted: 2022/04/18 | Published: 2022/05/28
References
1. Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015 Mar;4(1):204-47. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
2. Soleimani V, Delghandi PS, Moallem SA, Karimi G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytotherapy research. 2019 Jun;33(6):1627-38. [view at publisher] [DOI] [PMID] [Google Scholar]
3. Govind P, Sahni YP. A review on hepatoprotective activity of silymarin. Int J Res Ayurveda Pharm. 2011 Jan;2(1):75-9. [Google Scholar]
4. Zarif-Yeganeh M, Rastegarpanah M. Clinical role of silymarin in oxidative stress and infertility: A short review for pharmacy practitioners. Journal of research in pharmacy practice. 2019;8(4):181. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
5. Vahabzadeh M, Amiri N, Karimi G. Effects of silymarin on metabolic syndrome: a review. Journal of the Science of Food and Agriculture. 2018;98(13):4816-23. [view at publisher] [DOI] [PMID] [Google Scholar]
6. Mastron JK, Siveen KS, Sethi G, Bishayee A. Silymarin and hepatocellular carcinoma: a systematic, comprehensive, and critical review. Anti-Cancer Drugs. 2015;26(5):475-86. [view at publisher] [DOI] [PMID] [Google Scholar]
7. Gillessen A, Schmidt HH-J. Silymarin as supportive treatment in liver diseases: A narrative review. Advances in therapy. 2020;37(4):1279-301. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
8. Oufi HG, Al-Shawi NN, Hussain SA. What are the effects of silibinin on testicular tissue of mice? Journal of Applied Pharmaceutical Science.2012;2(11):9. [DOI] [Google Scholar]
9. Aghashahi M, Momeni HR, Darbandi N. Impact of aluminium toxicity on vital human sperm parameters-Protective effects of silymarin. Andrologia. 2020 Nov;52(10):e13742. [view at publisher] [DOI] [PMID] [Google Scholar]
10. Etemadi T, Momeni HR, Ghafarizadeh AA. Impact of silymarin on cadmium‐induced apoptosis in human spermatozoa. Andrologia. 2020 Dec;52(11):e13795. [view at publisher] [DOI] [PMID] [Google Scholar]
11. Rahimi-Madiseh M, Mohammadi M, Hassanvand A, Ahmadi R, Shahmohammadi M, Rostamzadeh A. Assessment of the toxicity effects of nicotine on sperm and IVF and the potential protective role of silymarin-an experimental study in mice. Middle East Fertility Society Journal. 2020;25:1-9. [view at publisher] [DOI] [Google Scholar]
12. Xie Y, Zhang D, Zhang J, Yuan J. Metabolism, transport and drug-drug interactions of silymarin. Molecules. 2019 Jan;24(20):3693. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
13. Heyat F. Cellular and molecular mechanisms of the production of free radicals during exercise and their function on skeletal muscles. Journal of Fasa University of Medical Sciences. 2017 Jun 10;7(1):1-1. [view at publisher] [Google Scholar]
14. Fanaei H, Azizi Y, Khayat S. A review: role of oxidative stress in male infertility. Journal of Fasa University of Medical Sciences. 2013;3(2):93-103. [view at publisher] [Google Scholar]
15. Alahmar AT. Role of oxidative stress in male infertility: An updated review. Journal of human reproductive sciences. 2019;12(1):4. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
16. Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. The Journal of nutrition. 2004 Dec 1;134(12):3431S-40S. [view at publisher] [DOI] [PMID] [Google Scholar]
17. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012;5(1):9-19. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
18. Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T, et al. Role of ROS and nutritional antioxidants in human diseases. Frontiers in physiology. 2018;9:477. [DOI] [PMID] [PMCID] [Google Scholar]
19. Selvam MKP, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radical Biology and Medicine. 2020;152:375-85. [view at publisher] [DOI] [PMID] [Google Scholar]
20. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Molecular reproduction and development. 2017;84(10):1039-52. [view at publisher] [DOI] [PMID] [Google Scholar]
21. Ramya T, Misro MM, Sinha D, Nandan D. Sperm function and seminal oxidative stress as tools to identify sperm pathologies in infertile men. Fertility and sterility. 2010 Jan 1;93(1):297-300. [view at publisher] [DOI] [PMID] [Google Scholar]
22. Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian journal of medical research. 2013 Feb 1;137(2). [view at publisher] [Google Scholar]
23. He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 2017;44(2):532-53. [view at publisher] [DOI] [PMID] [Google Scholar]
24. Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab journal of urology. 2019;17(2):87-97. [DOI] [PMID] [PMCID]
25. Saller R, Melzer J, Reichling J, Brignoli R, Meier R. An updated systematic review of the pharmacology of silymarin. Complementary Medicine Research. 2007;14(2):70-80. [view at publisher] [DOI] [PMID] [Google Scholar]
26. Jung YS, Kim SJ, Kim YS, Choi DW, Kim YC. Alterations in sulfur amino acid metabolism in mice treated with silymarin: a novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta medica. 2013;79(12):997-1002. [view at publisher] [DOI] [PMID] [Google Scholar]
27. Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clinical drug investigation. 2002;22(1):51-65. [DOI] [Google Scholar]
28. Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reproductive Biology and Endocrinology. 2003 Dec;1(1):1-6. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
30. Fatehi D, Mohammadi M, Shekarchi B, Shabani A, Seify M, Rostamzadeh A. Radioprotective effects of Silymarin on the sperm parameters of NMRI mice irradiated with γ-rays. Journal of Photochemistry and Photobiology B:Biology.2018;178:489-95. [view at publisher] [DOI] [PMID] [Google Scholar]
31. Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative medicine and cellular longevity. 2013;2013. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
32. Malmir M, Soleimani Mehranjani M, Naderi Noreini S, Faraji T. Protective antioxidant effects of N‐acetylcysteine against impairment of spermatogenesis caused by paranonylphenol. Andrologia. 2018;50(10):e13114. [DOI] [PMID] [Google Scholar]
33. Rabaça A, Ferreira C, Bernardino R, Alves M, Oliveira P, Viana P, et al. Use of antioxidant could ameliorate the negative impact of etoposide on human sperm DNA during chemotherapy. Reproductive biomedicine online. 2020;40(6):856-66. [view at publisher] [DOI] [PMID] [Google Scholar]
34. Shahrzad E, Shariati M, Naimi S, Edalatmanesh MA. Protective effect of N-acetylcysteine on changes in serum levels of Pituitary-Gonadal axis hormones and testicular tissue in acrylamide-treated adult rats. Advances in Human Biology. 2020;10(1):16. [view at publisher] [DOI] [Google Scholar]
35. Roostaei‐Ali Mehr M, Parisoush P. Effect of different levels of silymarin and caproic acid on storage of ram semen in liquid form. Reproduction in Domestic Animals. 2016;51(4):569-74. [DOI] [PMID] [Google Scholar]
36. Ziaei rad H, Rustaei Ali Mehr M, Mohammadi M. Effect of silymarin on rooster semen during storage at 4 C. Animal Science Researches. 2016;26(3):1-13. [Google Scholar]
37. Eskandari F, Momeni H. Effect of silymarin on DNA and nuclear integrity of ram sperm Treated with sodium arsenite. Journal of Cell & Tissue. 2016;7(4):429-36. [view at publisher] [Google Scholar]
38. Eskandari F, Momeni HR. Silymarin protects plasma membrane and acrosome integrity in sperm treated with sodium arsenite. International Journal of Reproductive BioMedicine. 2016;14(1):47. [DOI] [Google Scholar]
39. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. Journal of clinical and diagnostic research: JCDR. 2017;11(5):IE01. [DOI] [PMID] [PMCID] [Google Scholar]
40. Moshtaghion S-M, Malekinejad H, Razi M, Shafie-Irannejad V. Silymarin protects from varicocele-induced damages in testis and improves sperm quality: evidence for E2f1 involvement. Systems biology in reproductive medicine. 2013;59(5):270-80. [view at publisher] [DOI] [PMID] [Google Scholar]
41. Shafiei‐Roudbari SK, Malekinejad H, Janbaz‐Aciabar H, Razi M. Crosstalk between E2F1 and P53 transcription factors in doxorubicin‐induced DNA damage: evidence for preventive/protective effects of silymarin. Journal of Pharmacy and Pharmacology. 2017;69(9):1116-24. [view at publisher] [DOI] [PMID] [Google Scholar]
42. Nashwa A, Kawkab A, Mouneir S. The protective effect of ginger and N-acetyl cysteine on ciprofloxacin-induced reproductive toxicity in male rats. Journal of American Science. 2011;7(7):741-52. [Google Scholar]
43. Heidari Khoei H, Fakhri S, Parvardeh S, Shams Mofarahe Z, Ghasemnejad-Berenji H, Nazarian H, et al. Testicular toxicity and reproductive performance of streptozotocin-induced diabetic male rats: the ameliorating role of silymarin as an antioxidant. Toxin reviews. 2019;38(3):223-33. [DOI] [Google Scholar]
44. Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1546):1501-15. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
45. Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury‐associated testicular germ cell apoptosis. Proceedings of the Society for Experimental Biology and Medicine:Minireview.2000;225(2):105-15. [DOI] [Google Scholar]
46. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. International Journal of Reproductive BioMedicine. 2016;14(4):231. [DOI] [Google Scholar]
47. Abo El-Atta H, Ahmed D. Testicular dysfunction in malathion induced toxicity in male rats: Protective role of NAC and Silymarin. Mansoura Journal of Forensic Medicine and Clinical Toxicology. 2020;28(2):33-45. [view at publisher] [DOI] [Google Scholar]
48. Hamid AK, Ahmed MA, Tayawi HM. Silymarin effect as an antioxidant to improve damages induced by CCl4 on some characteristics of male rats reproductive system. Tikrit Journal of Pure Science. 2018;23(2):60-5. [view at publisher] [DOI] [Google Scholar]
49. Sheweita SA, Al-Shora S, Hassan M. Effects of benzo [a] pyrene as an environmental pollutant and two natural antioxidants on biomarkers of reproductive dysfunction in male rats. Environmental Science and Pollution Research. 2016;23(17):17226-35. [DOI] [PMID] [Google Scholar]
50. Yaman T, Uyar A, Kaya MS, Keles ÖF, Uslu BA, Yener Z. Protective effects of silymarin on methotrexate-induced damages in rat testes. Brazilian Journal of Pharmaceutical Sciences. 2018;54(1). [view at publisher] [DOI] [Google Scholar]
51. Khalil EA. Hormonal profile and histopathological study on the influence of silymarin on both female and male albino rats. The Egyptian Journal of Hospital Medicine. 2003;13(1):112-22. [view at publisher] [DOI] [Google Scholar]
52. Çeribaşı S, Türk G, Özçelik M, Doğan G, Çeribaşı A, Mutlu Sİ, et al. Negative effect of feeding with high energy diets on testes and metabolic blood parameters of male Japanese quails, and positive role of milk thistle seed. Theriogenology. 2020;144:74-81. [DOI] [PMID] [Google Scholar]
53. Chen Y-C, Pan L-C, Lai C-W, Chien Y-S, Wu T-H. Silymarin and protein kinase A inhibitor modulate glucose-mediated mouse sperm motility: An in vitro study. Reproductive biology. 2015;15(3):172-7. [DOI] [PMID] [Google Scholar]
54. El-Hady A, Mahmoud A, El-Tahawy NAE-L. The possible protective effect of vitamin E and∕ or silymarin on rat testes exposed to 950MHz electromagnetic field. Journal of Bioscience and Applied Research. 2015;1(3):97-111. [view at publisher] [DOI] [Google Scholar]
55. Abedi H, Jahromi HK, Hashemi S, Jashni HK, Jahromi ZK, Pourahmadi M. The effect of silymarin on spermatogenesis process in rats. Int Sci J Med Res Health Sci. 2016;5:146-50. [Google Scholar]
56. Attia YA, Hamed RS, Bovera F, Abd El AE-HE, Al-Harthi MA, Shahba HA. Semen quality, antioxidant status and reproductive performance of rabbits bucks fed milk thistle seeds and rosemary leaves. Animal reproduction science. 2017;184:178-86. [DOI] [PMID] [Google Scholar]
57. Al-Moziel MS, Kadhum HJ, Ghanim WK. EFFECTS OF TADALAFIL AND/OR SILYMARIN ON GONADAL FUNCTION IN ADULTS MALE ALBINO RATS. BasJVetRes. 2018;17(2):147-60. [Google Scholar]
58. McBride JA, Coward RM. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian journal of andrology. 2016;18(3):373. [DOI] [PMID] [PMCID] [Google Scholar]
59. Sahreen S, Khan MR, Khan RA. Ameliorating effect of various fractions of Rumex hastatus roots against hepato-and testicular toxicity caused by CCl4. Oxidative medicine and cellular longevity. 2013;2013. [view at publisher] [DOI] [PMID] [PMCID] [Google Scholar]
60. Faraji T, Momeni HR, Malmir M. Protective effects of silymarin on testis histopathology, oxidative stress indicators, antioxidant defence enzymes and serum testosterone in cadmium‐treated mice. Andrologia. 2019;51(5):e13242. [DOI] [PMID] [Google Scholar]
61. Malekinejad H, Janbaz-Acyabar H, Razi M, Varasteh S. Preventive and protective effects of silymarin on doxorubicin-induced testicular damages correlate with changes in c-myc gene expression. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2012;19(12):1077-84. [DOI] [PMID] [Google Scholar]
62. Rafiee F, Nejati V, Heidari R, Ashraf H. Protective effect of methanolic extract of Berberis integerrima Bunge. root on carbon tetrachloride-induced testicular injury in Wistar rats. International Journal of Reproductive BioMedicine. 2016;14(2):133. [DOI] [Google Scholar]
63. Mima M, Greenwald D, Ohlander S. Environmental toxins and male fertility. Current urology reports. 2018;19(7):1-8.doi: 10.1007/s11934-018-0804-1 [DOI] [PMID] [Google Scholar]
64. Goldstone AE, Chen Z, Perry MJ, Kannan K, Louis GMB. Urinary bisphenol A and semen quality, the LIFE Study. Reproductive Toxicology. 2015;51:7-13. [DOI] [PMID] [PMCID] [Google Scholar]
65. Wang Y-X, Zeng Q, Sun Y, You L, Wang P, Li M, et al. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: A cross-sectional study in China. Environmental research. 2016;150:557-65. [DOI] [PMID] [Google Scholar]
66. Eskandari F, Momeni HR. Protective effect of silymarin on viability, motility and mitochondrial membrane potential of ram sperm treated with sodium arsenite. International Journal of Reproductive BioMedicine. 2016;14(6):397. [DOI] [Google Scholar]
67. Choobineh T, Khodaei-Motlagh M, Momeni H, Darbandi N. Effect of silymarin and lithium chloride on DNA integrity in epidydimal ram sperm. Journal of Cell & Tissue. 2018;9(1):76-85. [view at publisher] [Google Scholar]
68. Choobineh T, Momeni H, Khodaei Motlagh M, Darbandi N, Khavari A. Study of Antioxidant Properties of Silymarin in Dealing with the Toxicity of Lithium Chloride in Ram Sperm. Iranian Journal of Animal Science Research. 2018;9(4):498-506. [Google Scholar]
69. Momeni H, Sepehri H, Yosefi M, Eskandari N. Protective effect of silymarin on viability, motility and mitochondrial membrane potential in spermatozoa treated with alominium. Journal of Cell & Tissue. 2018;9(2):102-11. [view at publisher] [Google Scholar]
70. Momeni HR, Sepehri H, Yosefi M. Effect of Silymarin on Plasma Membrane and Acrosome of Sperm Treated with Aluminum Chloride. Journal of Arak University of Medical Sciences. 2015;18(4):71-80. [Google Scholar]
71. Shah NA, Khan MR. Increase of glutathione, testosterone and antioxidant effects of Jurenia dolomiaea on CCl 4 induced testicular toxicity in rat. BMC complementary and alternative medicine. 2017;17(1):1-9. [DOI] [PMID] [PMCID] [Google Scholar]
72. Anderson D, Dobryńska M, Başaran N, Başaran A, Yu T-W. Flavonoids modulate comet assay responses to food mutagens in human lymphocytes and sperm. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1998;402(1-2):269-77. [DOI] [Google Scholar]
73. AL-Naimi RA. The therapeutic effect of Silybum marianum on the lead acetate induced-reproductive toxicity in both gender laboratory rats. Journal Of Wassit For Science & Medicine. 2012;5(1). [Google Scholar]
74. Zahra Z, Khan MR, Majid M, Maryam S, Sajid M. Gonadoprotective ability of Vincetoxicum arnottianum extract against bisphenol A‐induced testicular toxicity and hormonal imbalance in male Sprague Dawley rats. Andrologia. 2020;52(6):e13590. [DOI] [PMID] [Google Scholar]
75. Sajedianfard J, Nazifi S, Izadi A, Chahardahcherik M, Honarmand M. Effect of various doses of silymarin on the oxidative stress induced by busulfan administration in the different organs of rats. Turkish Journal of Pharmaceutical Sciences. 2016;13(2). [DOI] [Google Scholar]
76. Saleemi MK, Tahir MW, Abbas RZ, Akhtar M, Ali A, Javed MT, et al. Amelioration of toxicopathological effects of cadmium with silymarin and milk thistle in male Japanese quail (Coturnix japonica). Environmental science and pollution research. 2019;26(21):21371-80. [DOI] [PMID] [Google Scholar]
77. El-Sheshtawy R, El-Nattat W. Impact of silymarin enriched semen extender on bull sperm preservability. Asian Pacific Journal of Reproduction. 2017;6(2):81. [view at publisher] [DOI] [Google Scholar]
78. Marzban M, Anjamshoa M, Jafari P, Masoumi H, Ahadi R, Fatehi D. Effects of gamma rays on rat testis tissue according to the morphological parameters and immunohistochemistry: radioprotective role of silymarin. Electronic physician. 2017;9(6):4524. [DOI] [PMID] [PMCID] [Google Scholar]
79. Chen M-C, Lin J-A, Lin H-T, Chen S-Y, Yen G-C. Potential effect of advanced glycation end products (AGEs) on spermatogenesis and sperm quality in rodents. Food & function. 2019;10(6):3324-33. [view at publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







.png)
